
��14�֣���ѧ���о����ʵ���ɡ��ṹ�����ʼ��仯���ɵĿ�ѧ��
��1����ͭ���ڸɱ������Ȼ������������У������ӹ��ɵ��� ����д��ţ���ͬ�����������˹�������� ��
��2��ʳ���к��д��ᣨCH3COOH���������� ��Ԫ����ɣ���������⡢��ԭ�Ӹ�����Ϊ ��
��3������A������B�Ӵ��ɷ�����Ӧ�����ɹ���C��Һ��D�����۹���������ʾ��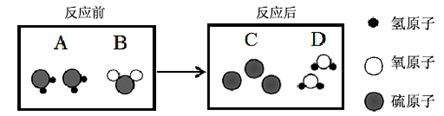
ͼ1 ͼ2
�� �÷�Ӧ�����У�������������� �������ƣ���
�� �����������ֻ����ƿ�зֱ�ʢ��A��B����ͼ2��ʾ����ʵ�顣��֪��ͬ�����£����������ȵ��ڷ��Ӹ����ȣ� ���ַ�Ӧ��ʣ��������� ���ѧʽ����
��4����ֽ�����������NaOH�ļ��Է�ˮ���辭���������Ժ��ŷš�
�� ��pH��ֽ����ˮ�ʼ��Եķ����� ��
�� ��ij��ֽ����ˮ�к�NaOH����������Ϊl.6%�����з�����9.8t��H2SO4����������Ϊ20%�������Դ����ķ�ˮ�����Ƕ��٣���д��������̣�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ���������dz��ý���п��ϡ���ᷴӦ����ȡ��������Ӧ�Ļ�ѧ����ʽΪ��Zn + H2SO4 = ZnSO4 + H2��
��1��С������ȡ8g���������������������Ҫ���Ķ��ٿ˵Ľ���п��
��2��������Ϊȼ�ϣ�Խ��Խ�ܵ����ǵĹ�ע��������Ϊȼ�ϵ��ŵ��Ǣ�
�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
Ԫ�ؼ��仯����֪ʶ�ǻ�ѧ�о���ѧϰ����Ҫ���ݡ�
��1���Ȼ��ơ��Ȼ��ƺ��Ȼ�þ�ж�������Ԫ�أ���ͼ������Ԫ�����ڱ��е������Ϣ������˵������ȷ���� �����ţ���

| A����Ԫ�����ڷǽ���Ԫ�� |
| B����ԭ�Ӻ���������Ϊ17 |
| C����Ԫ�ص�ԭ���γ������Ӻ����ӽṹʾ��ͼΪ |
| D����Ԫ�ص����ԭ������Ϊ35.45g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��NaCl ��Na2CO3�Ļ������Ʒ4g��Ϊ�˲ⶨ�û������Na2CO3�������������ֽ���������ӵ��˻�����У��õ�����������ͼ��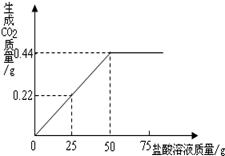
�� ��Ʒ��ȫ��Ӧ���ų�������̼���������
����Ϊ mol��
�� ����������Na2CO3������������
�����ݻ�ѧ����ʽ��ʽ���㣩
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
���ұ�����ʵ��С��ֱ���С�Na2CO3��NaOH�������Na2CO3�����ⶨ����ʵ�飺
(1)�����ʵ�鷽���ǣ���50�˵Ļ�����ܽ���ˮ�������Һ���μ�10%ϡ���ᣬ�۲쵽���� ����������������Һ���������ⶨ̼���Ƶĺ���������Ϊ��������Ƿ���ȷ,������_______ ��
��2������ͬѧ�ķ����ǣ����ݳ�������������ó�̼���Ƶĺ�����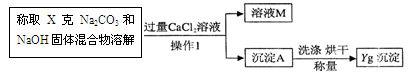
�Ҿ���ȷ���㣬�ó�̼���Ƶĺ���ƫ��ԭ������� ����ҺM�е����ʣ��û�ѧʽ��ʾ���� ��
��3�������ʵ�鷽���ǣ���50����Ʒ��������ϡ���ᷴӦ������ͼװ�òⶨ������CO2����������ͨ������ó���Ʒ��Na2CO3�������������װ�����Ͳ��������____ _ ____�����ռ���0��1Ħ��CO2���壬��ԭ�������Na2CO3�������ٷ����Ƕ��٣�����ȷ��0��1%���������ݻ�ѧ����ʽ��д��������̣�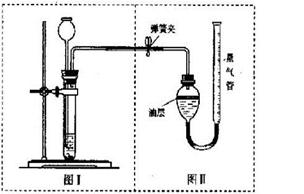
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
Ϊ���ijʯ��ʯ��Ʒ��̼��ƵĴ��ȣ�ȡ����Ʒ12g�����ձ��У������ձ��м���35gϡ���ᣬǡ����ȫ��Ӧ�����ʲ��μӷ�Ӧ������Ӧ���ձ���ʣ��������ʹ�42��6 g��
��1����Ӧ����CO2������Ϊ g��
��2��ʯ��ʯ��Ʒ��̼��Ƶ��������������������0��1%��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��9�֣�ҽ�ö�ˮ���Ȼ��ƿ����ڲ��ơ������������ȣ���ij̼�����Ʒ�����к�����Al3+��Fe3+�����ʣ�����ҽ�ö�ˮ���Ȼ��ƵĹ�������Ϊ�� ����֪��ˮ���Ȼ������¶ȳ���160��Cʱ�ֽ�Ϊ�Ȼ��ƺ�ˮ��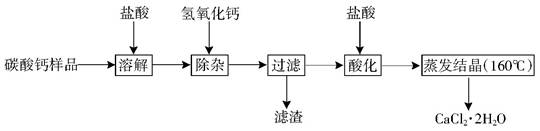
��֪���������ϵ�֪��������������ܽ�ʱ��pHΪ��
| �������� | Fe(OH)3 | Al(OH)3 | |
| ��ʼ����ʱ��pH | 2.3 | 4.0 | ��ʼ�ܽ⣺7.8 |
| ��ȫ����ʱ��pH | 3.7 | 5.2 | ��ȫ�ܽ⣺10.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��3�֣�Ϊ�ⶨʯ��ʯ��Ʒ��̼��Ƶĺ�����С��ȡ25 gʯ��ʯ��Ʒ��һ��������14��6%��ϡ����ǡ����ȫ��Ӧ������Ӧ���û�������ɵõ�����27��2 g�������ᾧˮ��������Ʒ�е����ʼȲ���ϡ���ᷴӦ�����Լ��㣺
��1���μӷ�Ӧ�������������
��2��ʯ��ʯ��Ʒ��̼��Ƶĺ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ʵ�����������һ��ʯ��ʯ������ͬѧ��ȥ�ⶨ����̼��Ƶ�����������ͬѧ����һ��������Ʒ�м���10%��ϡ���ᣬһֱ�ӵ����ٲ�������Ϊֹ������ȥϡ����73g��
��1������μӷ�Ӧ��̼��Ƶ�������
��2��ͬѧ�����������Ʒ��̼��Ƶ�����������ԭ���� __________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com