
 ��2010?��ƽ��2010�ꡰ����ˮ�ա��������ǡ������ˮ���������硱��
��2010?��ƽ��2010�ꡰ����ˮ�ա��������ǡ������ˮ���������硱��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2010?��ƽ����ɽ���к��и�Ũ�ȵķ�����ͼ��Ԫ�����ڱ��з�Ԫ�ص���Ϣʾ��ͼ��
��2010?��ƽ����ɽ���к��и�Ũ�ȵķ�����ͼ��Ԫ�����ڱ��з�Ԫ�ص���Ϣʾ��ͼ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
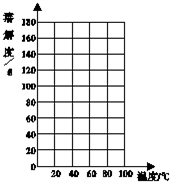 ��2010?��ƽ���±���������ڲ�ͬ�¶�ʱ���ܽ�ȣ�
��2010?��ƽ���±���������ڲ�ͬ�¶�ʱ���ܽ�ȣ�| �¶�/�� | 0 | 20 | 40 | 60 | 80 |
| �ܽ��/g | 13.3 | 31.6 | 63.9 | 110 | 169 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com