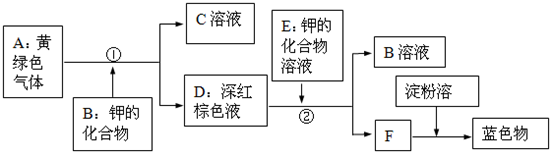
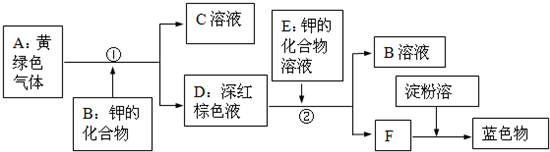
�Ķ��ش����⣺��ͨ��״���£�������һ��û����ɫû����ζ�����壬��101.3ǧ����-252.4������ɫ��Һ�壬-259.1��ʱ�ܱ��ѩ״�Ĺ��壮��������ˮ����״�����ܶ�Ϊ0.089g/L��������������ȼʱ���ڿ�����ȼ�գ���������ɫ���棬�ų��������ȣ�������������ȼʱ��������ը����˵�ȼ����ʱ��һ��Ҫ���������Ĵ��ȣ��������ܸ�������Cl2���ڵ�ȼ�������·�Ӧ�������Ȼ������壨HCl����
��1��ָ���������������ʣ� �� �� ������д���㣩
��2��ָ�������Ļ�ѧ����
��3����ǰ�������ٵ��н�������������Դ�����⣬����̸̸������Դ��Ϊδ��������Դ����ʶ�� ��
���𰸡���������1���������ʵ�����������ָ����Ҫͨ����ѧ�仯���ֳ��������ʣ�����������Ҫ�У���ɫ��״̬����ζ���ܶȡ�Ӳ�ȡ��۵㡢�е�Ƚ��н��
��2���������ʵĻ�ѧ������ָ�ڻ�ѧ�仯�б��ֳ��������ʣ���ѧ������Ҫ�У���ȼ�ԡ����ԡ������ԡ���ԭ�ԡ��ȶ��ԵȽ��н��
��3������Ŀǰʹ���������ڵ�����ش�
����⣺��1������������������ͨ��״������ɫ����ζ�����壻������ˮ���ܶ�Ϊ0.089g/L����101.3ǧ����-252.4C�����ɫ��Һ�壬-259.1Cʱ�ܱ��ѩ״�Ĺ��壻
��2�������Ļ�ѧ�����У������ڿ�����ȼ��������������ȼ�գ�˵���������п�ȼ�ԣ�
��3������ȼ������ˮ������Ⱦ����ֵ�ߣ�����������δ���������Դ������Ŀǰ������������д���ʹ������������һ�����ѣ���Ҫ�������������ȡ�ijɱ��ߺ��������ѣ�
�ʴ�Ϊ��
��1��ͨ��״������ɫ����ζ�����壻������ˮ���ܶ�Ϊ0.089g/L��
��2�����п�ȼ�ԣ�
��3��������δ���������Դ������Ŀǰ������������д���ʹ������������һ�����ѣ���Ҫ�������������ȡ�ijɱ��ߺ��������ѣ�
���������⿼�������������ʺ���;����ɴ��⣬�����������е�֪ʶ���У�Ҫ��ͬѧ�Ǽ�ǿ�Գ������ʵ�����ʶ�ǣ��Ա����Ӧ�ã�



 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�