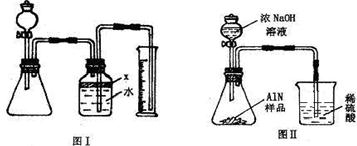
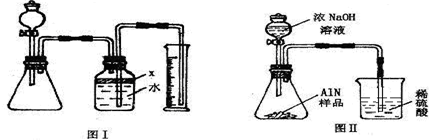
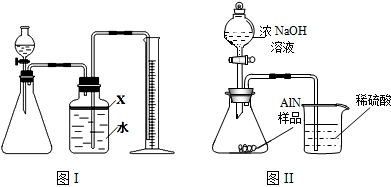
��������AlN����һ�����������ϣ��㷺Ӧ���뼯�ɵ�·��������ij�������к���̼�����������ʣ�����ͼ���е�һЩװ�������м��飬ʹ��������Ʒ��NaOH��Һ��ӦAlN+NaOH+H
2O=NaAlO
2+NH
3�������ݷ�Ӧ�������ɰ�����������ⶨ��Ʒ�еĵ�����������������������ʵ��������ȷ�����ʵijɷ֣�ʵ���е���������Բ��ƣ�
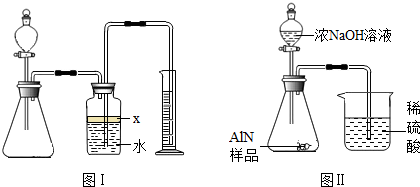
��1��ʵ���йز���Ϊ��a������ƿ�з���������AlN��Ʒ��b���ӷ�Һ©������ƿ�м��������ŨNaOH��c������װ�õ������ԣ�d���ⶨ�ռ���ˮ���������ȷ�IJ���˳��Ϊ
��
��2���������м��װ�������Եķ�����
��
��3�����ƿ�е��Լ�X��ѡ��
����ѡ��ı�ţ���A ���� B �ƾ� C ֲ���� D CCl
4��4�����ƿ��Һ��û��װ�����Ϸ����������ռ䣩��ʵ����NH
3�������
����ƫ��ƫС�䣩��
��5��ʵ����������۲쵽��ƿ�л��й��壬����Ʒ�к��е�������
��
��6����ʵ���в����Ʒ������Ϊwg�����������ΪaL������£�������Ʒ��AlN����������Ϊ
��AlN����Է�������Ϊ41��ÿ22.4L��������Ϊ17g����
��7�����˸���ͼ��װ�ý���ͬ��ʵ�飬ͨ���ⶨ�ձ��������������ȷ����Ʒ��AlN����������������Ϊ�Ƿ���У�
�����롰���С����������С�����ԭ����
���Ľ��ķ���Ϊ
��
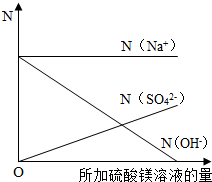
��8����mg 20%������������Һ�еμ�2��3�η�̪��Һ��Ȼ��߽������������м�������þ��Һ������Һ�ĺ�ɫ��ȫ��ȥʱ����ȥ�������Ƶ�ʣ����Һ������Ϊ3mg��
�ټ�����������þ��Һ������������
����N��ʾ��Һ�����ӵ���Ŀ����ͬ�����������ӷ���ע��[��N ��Na'����ʾ�����ӵ���Ŀ]���뽨������ϵ�����������μӹ����и������ӵ���Ŀ����Һ�IJ��ϼ�����仯�Ĺ�ϵͼ��





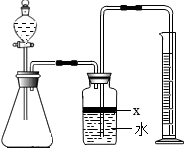 ��������AlN����һ�����������ϣ��㷺Ӧ���ڼ��ɵ�·��������ij�������к���̼�����������ʣ�ij��ѧ�о���ѧϰС����Ʋ����������ʵ�飮
��������AlN����һ�����������ϣ��㷺Ӧ���ڼ��ɵ�·��������ij�������к���̼�����������ʣ�ij��ѧ�о���ѧϰС����Ʋ����������ʵ�飮