
���������Ǵ�����Ⱦ��֮һ���ҹ��Ļ��������������жԿ����ж�����������Ũ�ȣ���λ����Ŀ��������������������������ֵ���ϱ���ʾ��
Ϊ�ⶨij�ؿ����ж�������ĺ�����ij��ѧ����С���������ʵ�飺���Թ��м���һ�����ĺ��⣨I2��1.27mg�ĵ���Һ���ټ���2~3�ε�����Һ��������I2����ɫ�������Թ���ͨ�����������Һ����ɫ��Ϊ��ɫʱǡ����ȫ��Ӧ������ȥ�������Ϊ1000L����ͨ�������жϳ��˿����ж��������Ũ�ȼ���
����ѧ����ʽΪ��SO2+I2+2H2O=H2SO4+2HI��
| Ũ����ֵ��mg/m3�� | ||
| һ���� | ������ | ������ |
| 0.15 | 0.50 | 0.70 |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
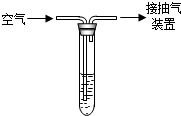 ���������Ǵ�����Ⱦ��֮һ���ҹ��Ļ��������������жԿ����ж�����������Ũ�ȣ���λ����Ŀ��������������������������ֵ�����ʾ��
���������Ǵ�����Ⱦ��֮һ���ҹ��Ļ��������������жԿ����ж�����������Ũ�ȣ���λ����Ŀ��������������������������ֵ�����ʾ��| Ũ����ֵ��mg/m3�� |
| һ���� ������ ������ 0.15 0.50 0.70 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
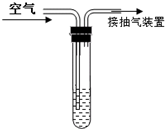 ���������Ǵ�����Ⱦ��֮һ���ҹ��Ŀ����������жԿ����ж�����������Ũ�ȣ���λ����Ŀ��������������������������ֵ���±���ʾ��
���������Ǵ�����Ⱦ��֮һ���ҹ��Ŀ����������жԿ����ж�����������Ũ�ȣ���λ����Ŀ��������������������������ֵ���±���ʾ��| ���� | һ��ָ�� | ����ָ�� | ����ָ�� |
| Ũ����ֵ��mg/m3�� | 0.15 | 0.50 | 0.70 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
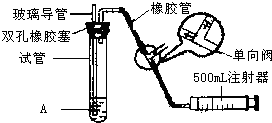 ���������Ǵ�����Ⱦ��֮һ��Ϊ���Եزⶨ��Χ�����е�SO2������ijѧ������С���������ͼ��ʵ��װ�ã�
���������Ǵ�����Ⱦ��֮һ��Ϊ���Եزⶨ��Χ�����е�SO2������ijѧ������С���������ͼ��ʵ��װ�ã�| S02���Ũ����ֵ����λmg?m-3�� | ||
| һ���� | ������ | ������ |
| 0.15 | 0.50 | 0.70 |
| ���� | ��һС�� | �ڶ�С�� |
| �������� | 120 | 140 |
| ������S02�ĺ�������λ��mg?m-3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
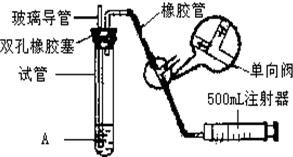 ���������Ǵ�����Ⱦ��֮һ��Ϊ���Եزⶨ��Χ�����е�SO2������ijѧ������С���������ͼ��ʵ��װ�ã�
���������Ǵ�����Ⱦ��֮һ��Ϊ���Եزⶨ��Χ�����е�SO2������ijѧ������С���������ͼ��ʵ��װ�ã�| S02���Ũ����ֵ����λmg?m-3�� | ||
| һ���� | ������ | ������ |
| 0.15 | 0.50 | 0.70 |
| ���� | ��һС�� | �ڶ�С�� |
| �������� | 120 | 140 |
| ������S02�ĺ�������λ��mg?m-3�� | 0.53 0.53 |
0.46 0.46 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com