��2005?��ݸ��ij����С���ͬѧ��ʯ�ҳ�����һ�麬���ʵ���ʯ������ȡ�Ȼ��ƣ����ȳƵ�����������Ϊ14g��Ȼ�������ʯ�ҷ����ձ��У������ձ��м���146g��������Ϊ10%��ϡ���ᣬǡ����ȫ��Ӧ�����ʲ���Ӧ��Ҳ������ˮ�����ѷ�Ӧ���Һ����ˣ���ֽ�����µĹ������ʾ�ϴ�ӡ������Ƶ���������Ϊ2.8g����������Ǽ��㣺
��1�������ʯ�ҵĴ��ȣ�______��
��2���ܹ��Ƶ��Ȼ��ƶ��ٿˣ�______��
���𰸡�
��������1���������ʲ���ϡ���ᷴӦ��ֻ�������ƺ�ϡ���ᷴӦ�����Կɸ��ݷ�Ӧ��ʣ��������������������Ƶ�������������������Ƶ�����������
��2��146g��������Ϊ10%��ϡ����ǡ������������ȫ��Ӧ���ɸ��ݻ�ѧ����ʽ���������Ȼ��Ƶ�������
����ⷨһ���������Ȼ��Ƶ�����Ϊx
CaO������=14g-2.8g=11.2g
��ʯ�ҵĴ���=

CaO+2HCl=CaCl
2+H
2O
56 111
11.2g x

�ⷨ������μӷ�Ӧ������������Ϊx�����ɵ��Ȼ�������Ϊy
CaO+2HCl�T�T�T�TCaCl
2+H
2O
56 73 111
x 146g×10% y

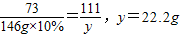
��ʯ�ҵĴ���=

�𣺣�1�������ʯ�ҵĴ�����80%����2���ܹ��Ƶ��Ȼ���22.2g��
�������������������������ͻ�ѧ����ʽ���ϵļ����⣬����������Ҫ��ȷд��������Ӧ�Ļ�ѧ����ʽ��Ȼ��Ҫ�ص����� �����ʼ�������ȣ�����һ��Ҫϸ��ȷ��







 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�