
a��2005��5��22������11ʱ08�֣��й���ɽ�����ӳɹ�������嶥�����Ǽ�1975����ҹ��ٴζ����������߶Ƚ��о�ȷ�������⻯�ƣ�CaH2�������ǵ�ɽ��Ա���õ���Դ�ṩ����������ˮ��Ӧ�����������ƺ�������CaH2+2H2O�TCa��OH��2+2H2��������ȼ��֮�裮����84g�⻯��������ˮ��Ӧ�������ɶ��ٿ�������
b��Ϊ�˲ⶨijͭп�Ͻ𣨼�ͭп������п������������ijͬѧ���øúϽ���ϡ����
��Ӧ������������ʵ�飬������ص�ʵ�����ݼ�¼���£�ʵ���е������Բ��ƣ���
| ��һ�� | �ڶ��� | ������ | |
| ��ȡ�Ͻ�������Mg | 25 | 25 | 50 |
| ����ϡ����������Mg | 120 | 160 | 100 |
| ���������������Mg | 0.4 | 0.4 | 0.4 |
��1���Լ����ͭп�Ͻ���п������������
��2�����ϱ����ݷ���������ȡ�Ͻ�������ϡ�����������Ϊ______ʱ�������Ͻ��е�п��ϡ�����е�����ǡ����ȫ��Ӧ��
��3�����ļ��η�Ӧ��������ʣ�ࣿ______��
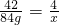
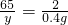
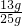 ��100%=52%��
��100%=52%��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��һ�� | �ڶ��� | ������ | |
| ��ȡ�Ͻ�������Mg | 25 | 25 | 50 |
| ����ϡ����������Mg | 120 | 160 | 100 |
| ���������������Mg | 0.4 | 0.4 | 0.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2008�꽭��ʡ�������п���ѧģ���Ծ��������������棩 ���ͣ������
| ��һ�� | �ڶ��� | ������ | |
| ��ȡ�Ͻ�������Mg | 25 | 25 | 50 |
| ����ϡ����������Mg | 120 | 160 | 100 |
| ���������������Mg | 0.4 | 0.4 | 0.4 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com