
��1�������������������ϵij������Ű��ֵȳ�������Ʒ��Ϊ�˷�ֹ�������⣬ͨ���ʺϲ���������һ�ַ�����������ţ�
�ڱ�������____���ڱ���Ϳ��____�ڱ����һ����������____��
��2���±���ij�ֳ��������IJ������ʣ�
��ɫ��״̬ | Ӳ�� | �ܶ� | �۵� | ������ | ������ | ��չ�� |
����ɫ���� | ���� | 2��70g/cm3 | 660��4�� | ���� | ���� | ���� |
���ý���Ͷ��ϡ�����У��ɲ�����������ɫ���塣�ش��������⣺
�����ƶϸý������ܵ�һ����;____��
���ý����Ļ�Ա�ͭ_____(����ǿ����������)��
��3��ijѧ����̽���������Ƿ����ͬʱ�п�����ˮ�������ɾ�����������A��B��C��֧�ɾ����Թ��У��ٸ�����ijЩ���ʻ���Ʒ�����о���
������ͼ��ÿ֧�Թ��л������������ӵ����ʻ���Ʒ��
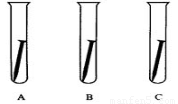
��һ�ܺ��Ϊ____���Թ��������������⡣
��д��һ����̼�ڸ��������°����ӳ�������Ҫ�ɷ������������ﻹԭ�����Ļ�ѧ����ʽ��____��
�����Ļ�Ա���ǿ�������ڿ�����ȴ���ֳ����õĿ���ʴ�ԣ���ԭ����____��
��ij�����ղ���̼3%������310t���ʸó�ÿ��������Ҫ��������80%�ij�����ʯ____��
��1�� �� ��? ��??? (2 ) �������ߡ����ߵ�?? ��ǿ
��3���� 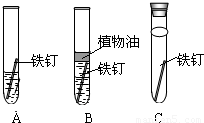 �� A? �� 3CO+Fe2O3
�� A? �� 3CO+Fe2O3 2Fe+3CO2
2Fe+3CO2
�����ڳ�����������е�������Ӧ���ڱ����γ�һ�����ܵ�������Ĥ? �� 536��9
��������
�����������1����ֹ������ʴ��ԭ��������������ˮ������������кܶ࣬���磺Ϳ�͡�ˢ�ᡢ�����ڱ�����������Ľ���������һ�����ڱ���ˢ�ᣬ�����ϵij�����Ϳ�ͣ��Ű�����Ϊ����Ҫ���˽Ӵ�������һ���ڱ����һ�����ʴ����������
��2�������ʵ����ʾ�����;����;��ӳ���ʣ����ݱ��е����ݣ�֪���ý����������õĵ����ԡ������ԡ���չ�ԣ����Կ����������ֽ����������ߡ����ߵȣ��ý���Ͷ��ϡ�����У��ɲ�����������ɫ���壬���Ի���������ʵ������ȡ�����������ý���Ͷ��ϡ�����У��ɲ�����������ɫ���塣˵��������ǰ����Ȼ��Ա�ͭǿ��
��3����̽�����������������������������ˮ������Ҫ��ƶԱ�ʵ�飺һ���Թ���װ���Թܵ�ˮ����Ҫʹ������һ�������ڿ����У��ڶ����Թ���װˮ��û����������������ֲ���ͽ����ܷ⣬һ�������Ǹ���Ŀ���(���)������Ϊ��������Ҫ������ˮ���������Թ�A�е����������⣬��Һ��һ�ֻ������ʷ�ɢ����һ��������γɾ�һ���ȶ��Ļ���������ҺҺ��СҺ�η�ɢ��Һ�����γɵĻ�������ϴ�Ӽ�����ϴ�ྫ��������ϴ���̡�ϴ��������ԡ¶�ȣ������黯���ܣ������ڳ�����������е�������Ӧ���ڱ����γ�һ�����ܵ�������Ĥ��������Ȼ���Ļ�Ա���ǿ�������ڿ�����ȴ���ֳ����õĿ���ʴ�ԣ������ݻ�ѧ����ʽ���㣺�����Ƶ�������������Ϊx ����������=310t����1-3%��=300��7t
3CO + Fe2O3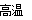 2Fe + 3CO2
2Fe + 3CO2
160??????? 112
X??????? 300��7t
160:112=X:300��7t
X=429��6t
�������������=429��6t��80%=536��9t
���㣺�������Ͻ�����ʺ���;,������������̽���Լ���ֹ�����ԭ������������ʩ���й�����ұ���Ļ�ѧ����ʽ�ļ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��4�֣�����������������������̼���Ҵ����밴����Ҫ����д��ѧʽ��
(1) ��ɫֲ����й���������յ���_____________
(2)�����ڹ�������ҽ�Ƽ��ȵ���___________
(3)�����ӵ���������Ϊ����ȼ�ϵ���
(4)������ʳƷ��װ�����Է�ʳƷ���ʵ��� ��
��(4��)�����������У���ᷢ�֡���ѧ�������ߡ���
��1��ϴ�ྫ��������ۣ�������������___ __���á�
��2���Ϻ������ڰ�װ�ġ�ֱ��ˮ���������á�����̿+���˲�+�����ߡ���ˮ���ա�����̿�ڴ���__ ___���ã��������ˮ����___ __��ѡ��������������
���ء���ٵ���Ŀ���˶�Ա�ڱ���ǰ���ð�ɫ�ġ�þ�ۡ����֣�������Ϊ��þ�ۡ����ᡢ��ˮ�Ժã���������������þ�ۡ�����Ч�ɷ��Ǽ�ʽ̼��þ��������ȼ��300�漴�ֽ⣬��ֽ�Ļ�ѧ����ʽ�ǣ�Mg5(OH)2(CO3)4 �� 5MgO+X+4CO2��,��X�Ļ�ѧʽ��____
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꽭��ʡ�γ���ѧ���꼶���ϣ���ĩ��ѧ�Ծ��������棩 ���ͣ������
 5MgO+X+4CO2������X�Ļ�ѧʽ�� ��
5MgO+X+4CO2������X�Ļ�ѧʽ�� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ��Ȫ���о��꼶���ϣ��¿���ѧ�Ծ��������棩 ���ͣ������
 5MgO+X+4CO2������X�Ļ�ѧʽ�� ��
5MgO+X+4CO2������X�Ļ�ѧʽ�� ���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com