
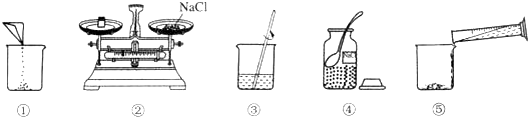
���� ��1������ͼ���õ��IJ������������з������
��2��������ƽ��ʹ��Ҫ��ѭ���������롱��ԭ�ݴ˽��з������
��3��������������=��Һ���������ʵ������������ɸ�����Һ�����������ʵ�������������������Һ����Ҫ�����ʵ��������ٸ����ܼ�����=��Һ����-�������������������ˮ�������������������ȷ��������Ͳ�����̣�
��4����������������������һ������Һ�Ļ���������з������
��5�������ܽ⡢���ˡ������в������IJ�ͬ���ý��з���
��6��������ƽ��ʹ�÷������н��
��7��Ϊ���ܹ������ʶ�����Һ��������ɺ�Ӧ���ϱ�ǩ���棻
��8�����������к�ǿ�ĸ�ʴ�ԣ�����ʱӦ���ڲ��������г��������Ӧ�ȳƳ��ձ����������ټ���������������Ƶij�����
��� �⣺��1��ѧ��Ӧ��ʶ���ò�����������ͼ�еIJ��������ֱ��ǹ��ƿ����Ͳ���ձ��Ͳ�������
��2��������ƽ����ҩƷӦ���������롱������ָ��ͼ�е�һ�����������ͼ�����Ȼ����������λ�õߵ���
��3����Ϊ����ó�����Ҫ��ˮΪ88mL��Ϊ�˾����������ѡ����88mL��������̣���������ʱӦѡ��100mL����Ͳ��ȡ����Ҫ��ˮ��
��4����Ϊ�������ʼ�ˮ�ܽ�����Ʋ���Ϊ����-����-�ܽ⣬����������ͼʾ����ű�ʾ������Һ�IJ���˳��ܢڢ٢ݢۣ�
��5�����ܽ��в������Ľ��������Ϊ�������Ȼ��Ƶ��ܽ⣻
��6���������룺���ʵ�����=���������+���������������������ʵ�����=���������-���������������С���Ƶõ��Ȼ�������ʵ��Ϊ5g-2.5g=2.5g��
��7��Ϊ���ܹ������ʶ�����Һ��������ɺ�Ӧ���ϱ�ǩ���棻
��8��Ϊ��ֹ�������Ƹ�ʴ��ƽ���̣�����ʱӦ���������Ʒ����ձ��н��г������ȳƳ��ձ���������Ȼ���������룬���������������Ƶ�������
�ʴ�Ϊ����1������������2��ͼ����������ҩƷ��λ�õߵ��ˣ� ��3��100mL�� ��4���ܢڢ٢ݢۣ���5�������Ȼ��Ƶ��ܽ⣻
��6��2.5g����7�����ñ�ǩ�� ��8���ձ������룮
���� ʹ�ù�����������Һ�IJ�������Ϊ����-����-�ܽ⣬ʹ��Һ������������Һ�IJ�������Ϊ����-��ȡ-�ܽ⣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Զ��ױ���18��ԭ�ӹ��� | |
| B�� | �Զ��ױ���̼Ԫ�ص���������ԼΪ90.6% | |
| C�� | �Զ��ױ������л��� | |
| D�� | �Զ��ױ���������ζ������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ֻ��Ag | B�� | ��Cu��Ag | C�� | ֻ��Fe | D�� | Ag��Fe��Cu |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H2CO3��NaCl��Mg��OH��2 | B�� | Ca��OH��2��HCl��Na2SO4 | ||
| C�� | MgSO4��H2SO4��HCl | D�� | CH3COOH��NaOH��Na2CO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ѧ�����һ����Ҫ����Դ�����������ʿɷ�Ϊ��һ�ε�أ�����ɵ�أ��Ͷ��ε�أ��ɳ���أ�����Ǧ�����أ������Ҫ�ش����и���
��ѧ�����һ����Ҫ����Դ�����������ʿɷ�Ϊ��һ�ε�أ�����ɵ�أ��Ͷ��ε�أ��ɳ���أ�����Ǧ�����أ������Ҫ�ش����и����鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com