
 ��ʵ������һƿ���õ��������ƹ��壬С��ͬѧ��̽��һ��
��ʵ������һƿ���õ��������ƹ��壬С��ͬѧ��̽��һ��
�ù���ijɷ֡������Ȳ��������ϣ���֪��
1.CaCl2+Na2CO3=2NaCl+CaCO3�� 2.CaCl2��Һ�����ԡ�
Ȼ��С����������ʵ�飺
��1��ȡ������Ʒ���Թ��У���ˮ�ܽ⣬Ȼ��μӷ�̪��Һ����Һ
��죬����Ʒ��һ�������������ƣ��ý��۲���ȷ��ԭ����
_______________________________________��
��2��ȡ������Ʒ������ϡ���ᣬ���������ɣ�����Ʒ��һ������____________��
��3��ȡ������Ʒ��ˮ�ܽ�ӹ����Ȼ�����Һ���а�ɫ�������ɣ����ˣ�����Һ�м����̪��Һ����죬�ò�ʵ���Ŀ����_________________________ __��
__��
������Һ��ͨ������������̼��������������____________����д����Ӧ�Ļ�ѧ����ʽ_______________________________________________________��
 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
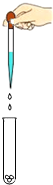 ��2012?������һģ����ʵ������һƿ���õ��������ƹ��壬С��ͬѧ��̽��һ�¸ù���ijɷ֣������Ȳ��������ϣ���֪��1��CaCl2+Na2CO3=2NaCl+CaCO3�� 2��CaCl2��Һ�����ԣ�
��2012?������һģ����ʵ������һƿ���õ��������ƹ��壬С��ͬѧ��̽��һ�¸ù���ijɷ֣������Ȳ��������ϣ���֪��1��CaCl2+Na2CO3=2NaCl+CaCO3�� 2��CaCl2��Һ�����ԣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�걱�����������п�һģ��ѧ�Ծ��������棩 ���ͣ�̽����
��5�֣���ʵ������һƿ���õ��������ƹ��壬С��ͬѧ��̽��һ��
�ù���ijɷ֡�
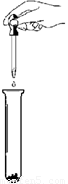
�����Ȳ��������ϣ���֪��
1.CaCl2+Na2CO3=2NaCl+CaCO3�� 2.CaCl2��Һ�����ԡ�
Ȼ��С����������ʵ�飺
��1��ȡ������Ʒ���Թ��У���ˮ�ܽ⣬Ȼ��μӷ�̪��Һ����Һ��죬����Ʒ��һ�������������ƣ��ý��۲���ȷ��ԭ����______ __________��
��2��ȡ������Ʒ������ϡ���ᣬ���������ɣ�����Ʒ��һ������____________��
��3��ȡ������Ʒ��ˮ�ܽ�ӹ����Ȼ�����Һ���а�ɫ�������ɣ����ˣ�����Һ�м����̪��Һ����죬�ò�ʵ���Ŀ����___________________________��
������Һ��ͨ������������̼��������������____________����д����Ӧ�Ļ�ѧ����ʽ_______________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʵ������һƿ���õ��������ƹ��壬С��ͬѧ��̽��һ��
�ù���ijɷ֡������Ȳ��������ϣ���֪��
1.CaCl2+Na2CO3=2NaCl+CaCO3�� 2.CaCl2��Һ�����ԡ�
Ȼ��С����������ʵ�飺
��1��ȡ������Ʒ���Թ��У���ˮ�ܽ⣬Ȼ��μӷ�̪��Һ����Һ��죬����Ʒ��һ�������������ƣ��ý��۲���ȷ��ԭ����_______________________________________��
��2��ȡ������Ʒ������ϡ���ᣬ���������ɣ�����Ʒ��һ������____________��
��3��ȡ������Ʒ��ˮ�ܽ�ӹ����Ȼ�����Һ���а�ɫ�������ɣ����ˣ�����Һ�м����̪��Һ����죬�ò�ʵ���Ŀ����___________________________��
������Һ��ͨ������������̼��������������____________����д����Ӧ�Ļ�ѧ����ʽ_______________________________________________________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʵ������һƿ���õ��������ƹ��壬С��ͬѧ��̽��һ��
�ù���ijɷ֡�
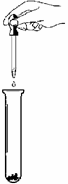
�����Ȳ��������ϣ���֪��
1.CaCl2+Na2CO3=2NaCl+CaCO3�� 2.CaCl2��Һ�����ԡ�
Ȼ��С����������ʵ�飺
��1��ȡ������Ʒ���Թ��У���ˮ�ܽ⣬Ȼ��μӷ�̪��Һ����Һ��죬����Ʒ��һ�������������ƣ��ý��۲���ȷ��ԭ����______ __________��
��2��ȡ������Ʒ������ϡ���ᣬ���������ɣ�����Ʒ��һ������____________��
��3��ȡ������Ʒ��ˮ�ܽ�ӹ����Ȼ�����Һ���а�ɫ�������ɣ����ˣ�����Һ�м����̪��Һ����죬�ò�ʵ���Ŀ����___________________________��
������Һ��ͨ������������̼��������������____________����д����Ӧ�Ļ�ѧ����ʽ_______________________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com