
Ϊ�ⶨ�Ȼ��ƺ�̼���ƹ����������Ȼ��Ƶ���������������������ʵ�鷽����
(1)����1��
���ʣ��ٲ������������ ��Ҫ������Ʒ���Ȼ��Ƶ�����������ʵ���б�������������ǣ�̼�ᱵ�������� ��
�����������ù���δ��ϴ�Ӽ���ɳ����������Ʒ���Ȼ��Ƶ����������� ��(�ƫ��ƫ�͡����䡱)
(2)����2��ͨ���ⶨ��Ʒ������������Һ��Ӧ���������������������Ʒ���Ȼ��Ƶ���������������ø����ʵ�����(g)����Ʒ����Ϊa������������Һ����Ϊb����ȫ��Ӧ������Һ����Ϊc�����������������Ϊ g��(��a��b��c��ʾ)

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ѡ��ǡ�����Լ�����ȥ�����ڵ����ʡ�
��1��CuCl2��HCl�� ����2��N2��CO2�� ����3��KOH��K2CO3�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ������������Һ�������õμӷ�ʽ��Ӧʱ����ҺpH�������Һ����仯�����ߡ�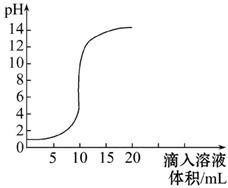
(1)�������ƺ�����ǡ����ȫ��Ӧʱ����Һ��pH 7(����ڡ���С�ڡ����ڡ�)��
(2)���������жϣ��÷�Ӧ�ǽ� (�����������Һ�������ᡱ����ͬ)���� �У������� ��
(3)��������Һ�����Ϊ5 mLʱ��������Һ�е�����Ϊ (д��ѧʽ)�����ڴ���Һ�е���ʯ����Һ����Һ�� ɫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ά����C(���Vc����������Ѫ��)��������ˮ���ױ�����������ȱ��Vc�����������ּ�����ˮ�����߲��к��зḻ��Vc��ij�о���ѧϰС�����̽�����£�
̽��һ���ⶨ������Vc�ĺ�����
���������ϡ�Vc�ܺ�����ط�Ӧ��ʹ��ɫ�ĸ��������Һ��ɫ��
����Ʒ������ֱ���ʢ��1 mL��Ũ�ȸ������ϡ��Һ����֧�Թ�����εμӹ�ζ���ϡ�ƻ��֭����֭��0.04%��Vc��Һ���ߵα���ֱ�����������Һ��ɫ��
��ʵ�����ݡ�
| | ��ζ���� | ƻ��֭ | ��֭ | 0.04%��Vc��Һ |
| �μӵĵ��� | 40 | 10 | 20 | 5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ˮ���д����������õĻ�ѧ��Դ�������Ȼ�þ���Ȼ��ơ��廯�صȡ��ۺ����ú�ˮ�Ʊ�����þ��������ͼ��ʾ��
(1)������Ҫ�ɷֵĻ�ѧʽ�� ��
(2)����a�������� ����ʵ�����н��д����������Ҫ�IJ����������ձ����������� ��
(3)��ҵұ��þ���õ��MgCl2�ķ�������ӦΪ��
MgCl2 Mg�� ��
Mg�� ��
(4)д���ڢڡ���������Ӧ�Ļ�ѧ����ʽ��
�� ��
�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ڻ������조�����Ļ�֮������ʳ�����������Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϡ�
(1)ijѧϰС����д����ᴿʵ�飬��Ҫ�������²������裺�������ܽ��_____���������ٴγ�����������ʡ�������������ʹ�õ���������������_________��
ʵ�������С�鷢������ʳ�β������Ե�������С�飬��ԭ�������_____��
A.����δ����ܽ����
B.�㵹ʱ���в����Ȼ�����Һ����
C.���������õľ��νϳ�ʪ
(2)��ⱥ��ʳ��ˮ���Եõ����ֻ�����Ʒ����Ҫ�������£�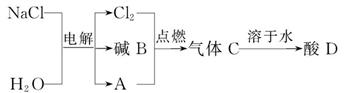
������A���ܶ���С�����壬�����������Ϊ________��д��һ������Dת��Ϊ����A�Ļ�ѧ����ʽ_____________________________________________��
�÷�Ӧ��___________(����ȡ������ȡ�)��Ӧ��
�ڵ������ɵļ�B�Ļ�ѧʽΪ_______��������������_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ϴ�Ӽ�(�������ϴ�·۵�)�������г��õ����ʣ�����Լ��ԡ�С��ȡ���������������ݵķ���ˮ����pH��ֽ���ԵĽ����pH 7(��д������ ����������)���������м�����������ɫ��̪��Һ(��ѧʽΪC20H14O4)������ˮ�� ɫ����̪���� ��Ԫ����ɵģ���̪������̼ԭ�Ӻ���ԭ�ӵĸ������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���к��Ȼ�þ���Ȼ������Ȼ����������ʵ���Һ�����ֻ�ñ��������ɳ���ʱ�����һ���ƵĻ�����仯ѧʽΪ��_____ �����Ҫ��þ���Ӻ�������ͬʱ���ɳ���ʱ�����һ���ƵĻ�����仯ѧʽ____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����ͼ��Ϣ��ƿ����Һ���ڷ��ú�Ũ�ȱ�ϡ��������Ϊ�����ʾ����� ���ԣ�������Ͳ��ȡ50ml�ܶ�Ϊ��g/ml��������Һ������Ͳ����Һ����������Ϊ�� ����ֻҪ����ʽ����
��2��ij̽��С��ͬѧ��ij��ҵ��ˮ������H2SO4��HNO3����H2SO4�ĺ������вⶨ��ȡ50g��ˮ���ձ��У���������BaCl2��Һ�����ˡ�ϴ�ӡ������BaSO4����11.65g����ش�
��50g��ˮ��H2SO4������������
��������KOH��Һ���ⶨ50g��ˮ��H2SO4�ĺ�����������ܻ��� ����ѡ�ƫ�͡�����ƫ�ߡ����䡱����ԭ���ǣ��� ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com