
 ��ǰ����ϵ�д�
��ǰ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2007?���ݣ�����ЧӦ������ȫ��㷺��ע��ijУ�о�С���ͬѧ����ѧ�������뻯ѧ����У�Ϊ���о�������CO2�����Կ����¶ȵ�Ӱ�죬����������ʵ�飺
��2007?���ݣ�����ЧӦ������ȫ��㷺��ע��ijУ�о�С���ͬѧ����ѧ�������뻯ѧ����У�Ϊ���о�������CO2�����Կ����¶ȵ�Ӱ�죬����������ʵ�飺| ʱ��/min �¶�/�� CO2���� | ��ʼ | 1 | 2 | 3 | 4 | 5 |
| ȫ������ | 23.0 | 23.3 | 23.9 | 24.6 | 25.0 | 25.5 |
| 10% | 23.0 | 23.5 | 24.2 | 25.0 | 26.0 | 27.0 |
| 20% | 23.0 | 24.0 | 24.5 | 25.6 | 26.5 | 27.5 |
| 30% | 23.0 | 24.5 | 25.0 | 26.2 | 27.5 | 29.0 |
| 100% | 23.0 | 30.0 | 33.0 | 35.6 | 37.0 | 39.5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��㶫ʡ�������п�һģ��ѧ�Ծ� ���������� ���ͣ�̽����
��7�֣�ijУ�����뻯ѧ���С����̽�����Ҫ��ȡһ�����İ�������С��������Ͽ�֪��
�ٳ�����NH3��һ����ɫ���д̼�����ζ�����壬��������ˮ���ܶȱȿ���С��
���Ȼ�粒������ʯ�ҷ�ĩ�ڼ������������ɰ���
2NH4Cl + Ca(OH)2 CaCl2 + 2NH3��+ 2H2O
CaCl2 + 2NH3��+ 2H2O
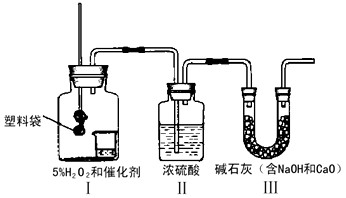
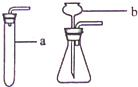
��С��1������������Ϣ������ͼ��ʾװ�ã�������пհף�
��ȡ��������װ���� ������ţ�����ȡ�����ռ�װ���� ������ţ���
��С��2��NH3ˮ��Һ�ʼ��ԣ���ʹʪ�����ɫ��ʯ����ֽ�����ɫ���ռ�����ʱ�����鰱�������ķ����ǣ�д��ʵ����������� ��
��С��3��NH3���л�ԭ�ԣ������������ܹ���ԭ����ͭ�õ�����ͭ���жϷ�Ӧ������������ ��
��С��4��Bװ�ÿ�����ʵ������ȡ�������仯ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com