
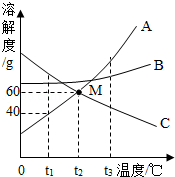
 ��ͼ�dz����������ʵ��ܽ�����ߣ�����ͼʾ�ش�
��ͼ�dz����������ʵ��ܽ�����ߣ�����ͼʾ�ش����� ���ݹ�����ܽ�����߿��ԣ��ٲ��ij������һ���¶��µ��ܽ�ȣ��Ӷ�ȷ�����ʵ��ܽ��ԣ��ڱȽϲ�ͬ������ͬһ�¶��µ��ܽ�ȴ�С���Ӷ��жϱ�����Һ�����ʵ����������Ĵ�С�����ж����ʵ��ܽ�����¶ȱ仯�ı仯������Ӷ��ж�ͨ�����½ᾧ���������ᾧ�ķ����ﵽ�ᴿ���ʵ�Ŀ�ģ�
��� �⣺��1��ͨ�������ܽ�����߿�֪��t3��ʱ��A��B��C�������ʵ��ܽ���ɴ�С��˳��A��B��C��
��2��t1��ʱ��A���ʵ��ܽ����40a�����Խ�10gA���ʷ���100gˮ�У�����ܽ�����õ���Һ�Dz�������Һ��C���ʵ��ܽ�����¶ȵ����߶���С��������ʹt2��ʱC���ʵı�����Һ��Ϊ��������Һ���ɲ�ȡ�ķ����ǽ����¶Ȼ��ˮ��
��3�����ܽ�����¶ȵĸı���ı䣬�����ڸò�������Һ�м�����������ͣ���������У��ܼ����������ܽ�ȣ���ѡ���٢ܣ�
���ܽ�����¶ȵĸı���ı䣬���ò�������Һ�������������ͣ�������������ʵ��������ܽ�ȣ���ѡ���ڢܣ�
�ʴ�Ϊ����1��A��B��C��
��2�������ͣ������¶Ȼ��ˮ��
��3���٢ܣ��ڢܣ�
���� �����ѶȲ��Ǻܴ���Ҫ�����˹�����ܽ����������ʾ�����壬�����ݹ�����ܽ�������������ص����⣬�Ӷ������������⡢��������������


 �ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
 ijͬѧ��һ������AgNO3��Cu��NO3��2�Ļ����Һ�м��˹���Zn����÷�Ӧ��������Һ���������Zn��������ϵ��ͼ��ʾ����ش��������⣺
ijͬѧ��һ������AgNO3��Cu��NO3��2�Ļ����Һ�м��˹���Zn����÷�Ӧ��������Һ���������Zn��������ϵ��ͼ��ʾ����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CaSiO4 | B�� | Ca2SiO3 | C�� | CaSi2O3 | D�� | CaSiO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ��ͼ��ij����ѧ��Ӧ����ģ��ͼ���ױ�ʾ��Ӧǰ��״̬���ұ�ʾ��Ӧ���״̬�����й��ڸ÷�Ӧ��˵����ȷ���ǣ�������
��ͼ��ij����ѧ��Ӧ����ģ��ͼ���ױ�ʾ��Ӧǰ��״̬���ұ�ʾ��Ӧ���״̬�����й��ڸ÷�Ӧ��˵����ȷ���ǣ�������| A�� | �÷�Ӧ�ǻ��Ϸ�Ӧ | B�� | �����������ҵ����� | ||
| C�� | �ұȼ�ԭ���������� | D�� | �ס��ҷ����������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CaSi04 | B�� | Ca2Si03 | C�� | CaSi203 | D�� | CaSi03 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  �㵹Һ�� | B�� |  ȡ�ù����ĩ | C�� |  ϡ��Ũ���� | D�� |  Ϩ��ƾ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������е���ë��ά������ά--���պ�����ζ | |
| B�� | �����̷������--�μӵ�Һ���۲���ɫ�ı仯 | |
| C�� | ����ʳ�κ��մ��--��ˮ�ܽ⣬�μ�ʳ�ף��۲������������� | |
| D�� | ����ˮ������ˮ--������������Һ���۲������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com