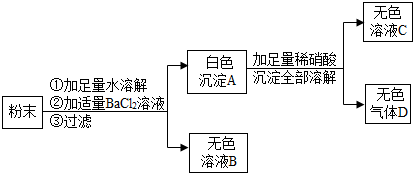
��2012?��������ģ���ڵ������������У����ɱ���ػ����һ�����ĺ�ͭ���ϣ��磺������£���ij��ѧ��ȤС����������ú�ͭ�����Ʊ�������CuSO
4?XH
2O����
�������ϣ���1�����������ڳ����»Ỻ���ֽ�Ϊ������ˮ������ˮ��Һ���ȡ����ջ�Ӵ�������MnO
2 ������ٽ���ֽ⣮
��2��CuSO
4?XH
2O������ˮ���ڼ���ʱ������CuSO
4��H
2O��
I������ͭ���Ʊ�
����1����ͬѧ������ѧ֪ʶ��������Ʊ�����ͭ������

����2����ͬѧ���ݲ��������ҵ���һ�ֹ�ҵ�Ʊ�����ͭ�����̣�
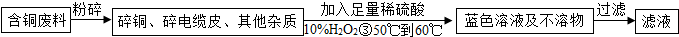
��1��д�����������еĢڴ�ͭ��ͭ�Ļ������йصĻ�ѧ��Ӧ����ʽ�ǣ�
CuO+H2SO4=CuSO4+H2O
CuO+H2SO4=CuSO4+H2O
��
��2���ӻ����Ƕȶ��������IJ�ͬ���ֽ��бȽϣ�����Ϊ����
2
2
����1��2���������������ǣ�
�����ٻ�����Ⱦ
�����ٻ�����Ⱦ
��
��3������2������H
2O
2��Ҫ���¶ȿ�����50�浽60����ȵĿ��ܵ�ԭ��Ϊ
��ֹH2O2���ȷֽ�
��ֹH2O2���ȷֽ�
��
��4���������������õ�ϡ��������ʵ��������ϡ��Ũ����õ�����ϡ��Ũ����ʱ�IJ����ǣ�
��Ũ�������ձ��ڻ�������ˮ��
��Ũ�������ձ��ڻ�������ˮ��
���ò��������Ͻ���
���ò��������Ͻ���
��
��5����ȤС��ͬѧһ��ָ��������Һ�����������½ᾧ�Ȳ�����������95%�ľƾ���ϴ�����ɣ�������ͭ���壮������þƾ���ϴ���ŵ���
��������ͭ����������ˮ����ʧ
��������ͭ����������ˮ����ʧ
��
II������ͭ���壨CuSO
4?xH
2O���нᾧˮ��xH
2O�������IJⶨѧϰС��ȡ12.5g����ͭ���壨CuSO
4?xH
2O�������ȷֽ⣬
���������ݣ������Ƴɹ�������һ�¶ȵĹ�ϵ��ͼ��
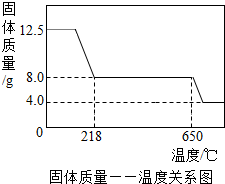
��1���ڼ��ȹ����У�����ˮ�ֵ�ʧȥ��������ɫ������ɫ��Ϊ
��
��
ɫ�����ձ�Ϊ��ɫ��
��2��650������ʱ������ͭ����ͻᷢ���ֽ⣬���ɺ�ɫ������������������������˷�Ӧ�Ļ�ѧ����ʽΪ
��
��3������ͼ�����ݣ�����CuSO
4?xH
2O��x��ֵΪ
5
5
������д��������̣�
��4������4.0gʣ�����������ȵ����ߵ��¶ȣ����ֹ�������������0.4g���������ٸı䣬д��ʣ�����Ļ�ѧʽ��
Cu2O
Cu2O
��
������ͭ��Ӧ��
������Һ��������ͭ��ʯ������ɵ�һ������ɫ��ճ��������Һ������Ч�ɷ���Cu
4��OH��
6SO
4������ͭԪ�صĻ��ϼ�Ϊ
+2
+2
����ʽ�ο���д���κͼ����ʽ�������ʽ̼��ͭ����д��CuCO
3?Cu��OH��
2����Cu
4��OH��
6SO
4����д���κͼ����ʽΪ
CuSO4?3Cu��OH��2
CuSO4?3Cu��OH��2
����ɱ�������ɿ�����Cu
2+ʹ��ԭ��ʧȥ���ԣ���˵����ԭ����������
������
������
����һ���л������ƣ���





 ��У����ϵ�д�
��У����ϵ�д�