��2007?���Ƹ�ģ�⣩ij��һѧϰС���ԡ������г��ϼ��ֲ��Ʊ�����Ʒ�ĵ����о���Ϊ���������̽��ѧϰ�������һ����������������̽�����̣�
[�Ŀ��]
��1���˽��Ԫ�������彡���Ĺ�ϵ��
��2���˽�Ʋ�Ʒ�ĺ��������۸������Ƿ�ϸ�
��3��������ȡ��Ϣ��������Ϣ���������⡢������⡢����̽��������
[�����ռ�����]
��1��ͨ���г����飬���������ָƲ�Ʒ���й���Ϣ���£�
| Ʒ�� | �ƶ���-D | ��˼��-D | ������ | ������� |
| ��Ҫ�������� | ̼��� | ̼��� | ���������ϸ� | ������� |
| ��Ԫ�غ�����mg/Ƭ�� | 600 | 500 | 250 | 168 |
| ������Ƭ��/ƿ���У� | 30 | 20 | 30 | 30 |
| �۸�Ԫ��/ƿ���У� | 27.00 | 23.10 | 30.00 | 25.20 |
��2��ͨ���������Ž����������ִ�ҽѧ�ܿ��������г��������ռ���������Ϣ��
������������ÿ���������ӦΪ1 300 mg����ʵ����Ŀǰ�ҹ���ѧ��ÿ�ո��������ľ�ֵΪ518 mg��Ӫ��ѧ�ṫ���ľ���ÿ�ո�������Ϊ800 mg��
[�����]
��1���Ʊ�����Ʒ�еĸ�Ԫ����______��ʽ���ڣ� A������B��������
��2������ĸ�Ԫ����Ҫ������______�У�
��3����������������Ϣ������������ÿ�ո���Ҫ����ʵ�����������㣬Ӧע��______��
[����������]
��1�����ϱ������Ƚϣ����ָƲ�Ʒ�У���������ͬ�����ĸ�Ԫ��ʱ�۸���������______��
A���ƶ���-D B����˼��-D C��������D���������
��2���ڽ��������У�С��ͬѧ���һ���۵㣬���ƻ�����ʳ��������ʡǮ���ܲ��ƣ������������Բ��Ƶ���______��
A����ʳ����������ʯ��B��ʳ��һЩϺƤC�����Ź���D������ͷʱ�Ŵ���
��3��С����Ϊʳ�ÿ�˼��-D��̼��ƽ���θ���ܷ�����ѧ��Ӧ���仯ѧ����ʽ�ǣ�______��
[��������̽��]
�ɸƶ���-D˵����֪ÿƬ�ƶ���-D�к�̼���1.5 g������֪���ƶ���-D����������̼���Σ�����ϸ����ϡ������ȫ��Ӧ�����������У�Сǿͬѧ���һ�����⣬�����г�����Щ�������ӻ��Լ����棬����ĸƶ���-D��֪�Ƿ�ϸ���ͨ��ʵ�ʶ���������Сǿ��������·���������ʵ�飺ȡ50Ƭ�ƶ���-D��ϸ��������ƿ������ͼ�������������������ַ�Ӧ���������ͨ������������Һ����������ȫ���������������գ�����ϵͳ�ڿ�����CO
2��Ӱ�죩�����γ���ʢ����������Һ��ϴ��ƿ�ɼ������Ʒ�Ƿ�ϸ��Իش��������⣺
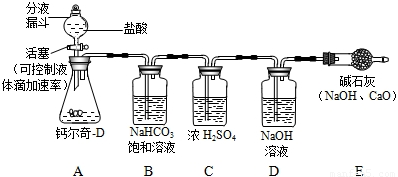
��1��Сǿ��B��NaHCO
3������Һ��ȥCO
2�л��е�����HCl���壬B�е�NaHCO
3��Һ�ܷ�ʯ��ˮ������ܡ�������������______��
��2��С����Ϊ��Сǿ����Ʋ��ܴﵽ������Ʒ�Ƿ�ϸ��Ŀ�ģ�Ӧ�ü��ԸĽ������õ���С������ͬѧ����ͬ������ΪС�����ֵ�������______���Ľ��ķ�����______��
��3���øĽ����װ�ú�ҩƷ����ʵ�飬����ʢ����������Һ��ϴ��ƿ������33 g����ͨ������˵��Сǿѡ�õĸƶ���-D�Ƿ�Ϊ�ϸ��Ʒ��




 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�