
| ����ʱ��/min | 2 | 4 | 6 | 8 | 10 |
| ��������/g | 44 | 39 | 35 | 32 | 32 |
| ��������/g | 2 | 7 | 11 | 14 | X |
 Cu+CO����2CuO+C
Cu+CO����2CuO+C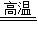 2Cu+CO2������̿�ۺ�����ͭ�������������Ȼ�Ϻ��������ʵ�飬��������ΪCO��CO2����______������ţ���
2Cu+CO2������̿�ۺ�����ͭ�������������Ȼ�Ϻ��������ʵ�飬��������ΪCO��CO2����______������ţ���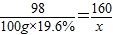
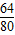 =32g�����Թ�������Ϊ32gʱ��Ӧ�Ѿ���ȫֹͣ�����������������Ӧ����Ϊ14g��
=32g�����Թ�������Ϊ32gʱ��Ӧ�Ѿ���ȫֹͣ�����������������Ӧ����Ϊ14g��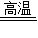 Cu+CO����2CuO+C
Cu+CO����2CuO+C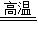 2Cu+CO2�������Կ���ֻ����һ����̼ʱ����ͭ��̼������֮��20��3��ֻ���ɶ�����̼ʱ����ͭ��̼������֮��40��3������BC��������ϵ��Ӧʱ���ɵ�������һ����̼�Ͷ�����̼ͬʱ���ڣ�
2Cu+CO2�������Կ���ֻ����һ����̼ʱ����ͭ��̼������֮��20��3��ֻ���ɶ�����̼ʱ����ͭ��̼������֮��40��3������BC��������ϵ��Ӧʱ���ɵ�������һ����̼�Ͷ�����̼ͬʱ���ڣ�

 ����˼ά�żӿ���ϵ�д�
����˼ά�żӿ���ϵ�д� �����Ծ�ϵ�д�
�����Ծ�ϵ�д� �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ����ʱ��/min | 2 | 4 | 6 | 8 | 10 |
| ��������/g | 44 | 39 | 35 | 32 | 32 |
| ��������/g | 2 | 7 | 11 | 14 | X |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ����ʱ��/min | 2 | 4 | 6 | 8 | 10 |
| ��������/g | 44 | 39 | 35 | 32 | 32 |
| ��������/g | X | 7 | 11 | 14 | 14 |
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ����ʱ��/min | 2 | 4 | 6 | 8 | 10 |
| ��������/g | 44 | 39 | 35 | 32 | 32 |
| ��������/g | 2 | 7 | 11 | 14 | X |
 Cu+CO����2CuO+C
Cu+CO����2CuO+C 2Cu+CO2������̿�ۺ�����ͭ�������������Ȼ�Ϻ��������ʵ�飬��������ΪCO��CO2����______������ţ���
2Cu+CO2������̿�ۺ�����ͭ�������������Ȼ�Ϻ��������ʵ�飬��������ΪCO��CO2����______������ţ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��㶫ʡ�п���ѧ�Ծ��������棩 ���ͣ������
| ����ʱ��/min | 2 | 4 | 6 | 8 | 10 |
| ��������/g | 44 | 39 | 35 | 32 | 32 |
| ��������/g | X | 7 | 11 | 14 | 14 |
 Cu+CO����2CuO+C
Cu+CO����2CuO+C 2Cu+CO2���������ܷ�����Ӧ�Ļ�ѧ����ʽΪ______ 2CO
2Cu+CO2���������ܷ�����Ӧ�Ļ�ѧ����ʽΪ______ 2CO�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com