
���ж��������¹ʵ�Ԥ������������ǣ�������
ѡ�� �����¹� Ԥ����������
A �Թ��е�Һ�屩�� �������ԹܴӾƾ��ƻ������ƿ�
B ������ȼ�ŵľƾ���������
�����ľƾ�������ȼ�� Ӧ������ʪĨ����ɳ�˸�
C Ũ���������Ƥ���� ���øɲ���ȥ��Ȼ���ô���ˮ��ϴ��
��Ϳ��3%��5%��С�մ���Һ
D ��Һ���������·��� ��ˮ��ϴ����Ϳ��������Һ
A��A B��B C��C D��D
���㡿�����������¹ʵĴ�����������������-�ƾ��ƣ����Թ����Һ����ȣ�
��ר�⡿��ѧѧϰ�е�ʵ��˼�룻������������ѧʵ�����������
��������A�������Թ��е�Һ�屩�еĴ����������з����жϣ�
B�����ݾƾ��Ƶ�ʹ�÷������з����жϣ�
C������Ũ���������Ƥ���ϵĴ����������з����жϣ�
D�����ݼ�Һ���������·��ϵĴ����������з����жϣ�
����𡿽⣺A���Թ��е�Һ�屩�У��������ԹܴӾƾ��ƻ������ƿ�����ѡ�������¹ʵ�Ԥ��������ȷ��
B��������ȼ�ŵľƾ��������������ľƾ�������ȼ�գ�Ӧ������ʪĨ����ɳ�˸ǣ��Ը��������ﵽ����Ŀ�ģ���ѡ�������¹ʵ�Ԥ��������ȷ��
C��Ũ���������Ƥ���ϣ����øɲ���ȥ��Ȼ���ô���ˮ��ϴ����Ϳ��3%��5%��С�մ���Һ����ѡ�������¹ʵ�Ԥ��������ȷ��
D����Һ���������·��ϣ�����ˮ��ϴ�ɣ�����Ϳ��������Һ��������и�ʴ�ԣ�����ѡ�������¹ʵ�Ԥ����������
��ѡ��D��
�������������ѶȲ������ճ��������¹ʵĴ���������������ѧʵ�������ע�����������ȷ�����Ĺؼ���
��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijͬѧ����60g������������Ϊ5%��NaCl��Һ��������ͼ1��ʾʵ����Ʒ���ش��������⣺
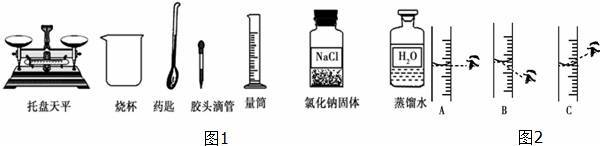

��1����ͬѧ������ʵ�鲽����У��ټ��㣬�ڳ���������ȡ������������������ƣ�
��2�����ƹ��̻�ȱ�ٵ�һ�ֲ����������������������ƣ�
��3����������NaCl����Ϊ3g������ʱNaClӦ����������ƽ������������̡������̡����ϣ�
��4����ȡ����ˮ�������£�
������ˮע����Ͳ����Һ��ӽ���ȡ�����Ӧ�̶���ʱ���������������������ƣ��μ�����ˮ���̶��ߣ���ͼ2��ʾ����ͬѧ�۲췽ʽ��ȷ������������ţ���
��5������C�۲췽ʽ��ȡˮ���������������Һ�����������������������ƫ��ƫС������Ӱ�족����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
θ����Ҫ�ɷ������ᣬ������θҺÿ����Ҫ������������ԼΪ6.5g��7.3g��ij����ÿ�շ���������������Ϊ9.0g��Ϊ����θ����������ͼ9��ʾ�����������㲢�ش𣺰�˵�����÷�������ÿ�������к�θҺ���������������Ƕ��ٿˣ��������ܷ�ʹ�û���θҺ���������������ָ���������Χ����д��������̣������ȷ��0.001��


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ˮ����������������������أ���ش��������⣺
��1��ͼ����ˮͨ��ֽ��ʾ��ͼ����ʵ������У��Թ�a�в���������������������������2��ij�з�������ʱ��������Ա�ø�ѹˮǹ��𣬴����ԭ�������� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��һ������Ũ����Ĺ�����������������������Ϊ98%��Ũ����20tй©������·���������ӡ��ӵ������� �����ٱ�������������ʯ�ҽ�����Ҫ�ɷ�Ϊ�������ƣ��к������������顣�÷�Ӧ�Ļ�ѧ����ʽΪ��H2SO4 + Ca(OH)2 === CaSO4 + 2H2O
�����ٱ�������������ʯ�ҽ�����Ҫ�ɷ�Ϊ�������ƣ��к������������顣�÷�Ӧ�Ļ�ѧ����ʽΪ��H2SO4 + Ca(OH)2 === CaSO4 + 2H2O
��ش�
��1��������������Ϊ98%��Ũ����20t�к�H2SO4�������� ��
��2�������к�й©��98%Ũ����20t����������Ҫ�������Ƶ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ�����������dz������Σ���ش��������⣮
��1����̼������Һ�е����Ȼ�����Һ��������������
��2��̼��ơ�̼���ƾ�����ϡ���ᷴӦ����CO2������Ϊ̼��ơ�̼�����о�����������д���ӷ��ţ���
��3������ͭ��Һ������������Һ�ܷ�����Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ��������ͭ��Һ���Ȼ�����Һ���ܷ�Ӧ������������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ճ�ʹ�õĽ������ϣ���������ںϽ𣮻�ͭ����п����Ҫ����Ԫ�ص�ͭ�Ͻ�
��1����ͭ��Cu2O�����ҹ��Ŵ���ȡ��ͭ��һ��ԭ�ϣ� Cu2O��ͭԪ������Ԫ�ص���������������
��2���������쵯�ǵĻ�ֻͭ����п��ͭ����42.0g���Ƿ���ʢ��200gϡ������ձ��У�����
�������������Dz����ܽ���ձ��л�����������241.6g�����㣺
�ٵ�����ͭ��������
�ڷ�Ӧ��������Һ��ZnSO4����������������������һλС������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijͬѧ����Ͳ��ȡҺ�壬�������Ӷ�����19�������������Һ����ٸ��Ӷ�����11���������ͬѧ�����Һ���ǣ�
A. 8���� B. ����8���� C. С��8���� D. ���ж�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����У���ȥ���������������ʵķ����ͷ�Ӧ���������ȷ����( )
| ѡ�� | ���� | �������� | ��ȥ���� | ��Ӧ���� |
| A | CuO�� | Cu�� | �ڿ����м��� | ���Ϸ�Ӧ |
| B | CO2 | CO | ͨ�����ȵ�CuO | �û���Ӧ |
| C | H2 | HCl | ͨ��ʢ����������Һ��ϴ��ƿ | �ֽⷴӦ |
| D | KCl | K2SO4 | �ܽ⣬�����������ᱵ��Һ�������� | ���ֽⷴӦ |
A��A B��B C��C D��D
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com