
 С��ͬѧ���ڼ�����ȡһЩ����������Һ������ʢ�г���ʯ��ˮ���ձ�������ؼ�����һЩ̼������Һ�����ɰ�ɫ���������˺�õ���ɫ��Һ����˵�������Ƶ�������������Һ��������������ʵ��֤�����Ĺ۵�
С��ͬѧ���ڼ�����ȡһЩ����������Һ������ʢ�г���ʯ��ˮ���ձ�������ؼ�����һЩ̼������Һ�����ɰ�ɫ���������˺�õ���ɫ��Һ����˵�������Ƶ�������������Һ��������������ʵ��֤�����Ĺ۵�| ʵ�鲽�� | ʵ������ | ʵ����� |
| ȡ����������Һ�������еμ�������̼������Һ | �ް�ɫ�������� |
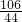 =
= X=2.12��
X=2.12�� ��100%=2.12%
��100%=2.12%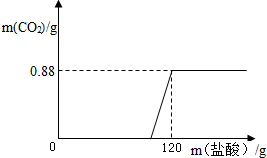


 ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д�
ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д� Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 С��ͬѧ���ڼ�����ȡһЩ����������Һ������ʢ�г���ʯ��ˮ���ձ�������ؼ�����һЩ̼������Һ�����ɰ�ɫ���������˺�õ���ɫ��Һ����˵�������Ƶ�������������Һ��������������ʵ��֤�����Ĺ۵�
С��ͬѧ���ڼ�����ȡһЩ����������Һ������ʢ�г���ʯ��ˮ���ձ�������ؼ�����һЩ̼������Һ�����ɰ�ɫ���������˺�õ���ɫ��Һ����˵�������Ƶ�������������Һ��������������ʵ��֤�����Ĺ۵�| ʵ�鲽�� | ʵ������ | ʵ����� |
| ȡ����������Һ�������еμ�������̼������Һ | �ް�ɫ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
| ʵ�鲽�衡 | ��ʵ������ | ʵ����ۡ� |
| ȡ����������Һ�������еμ�����̼������Һ�� | ���ް�ɫ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009�꽭��ʡ�����н�̳�о��꼶�������л�ѧ�Ծ��������棩 ���ͣ������
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ȡ����������Һ�������еμ�������̼������Һ | �ް�ɫ�������� |

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com