
Ŀǰȫ������������Դ��������ˮ��Դ����ʳ�ȷ����Σ����
��1��Ŀǰ����ͨ����ѧ��Ӧ��õ�����������Ի�ʯȼ�ϣ���������½��ʹ����Ȼ����д����Ȼ���ڿ�������ȫȼ�յĻ�ѧ��Ӧ����ʽ��
����ʯȼ�ϵ�ʹ�ã������ǵ���������ܶ�ķ��㣬ͬʱҲ�Ի�������˲���Ӱ�죮�磺úȼ��ʱ���ŷų���������SO
2��������������NO
2������Ⱦ�úȼ��ͬʱ����˿����еĶ�����̼��Ũ�ȣ�������
����ЧӦ
����ЧӦ
����̼������ָ������Ҫ���������������ĺͲ������ģ��Ӷ����Ͷ�����̼���ŷ����������������ϵ�̼�����������
ABD
ABD
��������ĸ��ţ�
A������һ���Ե�ľ�� B�����������ε���ͷ
C��������չ�������� D���Ż�������ƣ��������ͱ��²���
��2����ˮ������֮Դ�����������ȹ����Ӳ�ȹ����ˮ�����������彡��������ˮ��Ӳˮ������ˮ�����õ�������
����ˮ
����ˮ
������˵��������ʹӲˮ������һ�ֳ��÷���
�������
�������
��
��3������ˮ��Դ�ļ��٣���ʳ���������ܵ�����Ӱ�죮��ʳ�е���Ҫ�ɷ�Ϊ���ۣ���������
����
����
��������ʡ���ά���ء�����֬�������ࡱ������������������Ҫ��Դ��
��4����2011��5��1���𣬼�ʻԱ������ݳ�Ҫ���������Σ���˾����ƾ�����Ǵ�����ͨ����ʾ����ɫ�仯���ɲ��˾���Ƿ�ƺ�ݳ����仯ѧ��Ӧԭ�����£�C
2H
5OH+4X������ɫ��+6H
2SO
4=2Cr
2 ��SO
4��
3����ɫ��+2CO
2��+9H
2O����X�Ļ�ѧʽΪ
CrO3
CrO3
��5����������������pHΪ5��6�����������У��ڲ������������У�Ϊ�˷��β��溦������ʱ����ũҩ������Һ����ʯ�Һ�����ͭ�Ļ��Һ��������IJ��ܹ�ʵ����ɿڣ������������ϻش��������⣺
A����������������
����
����
������ԡ��������ԡ������ԡ����������
B�����²�����ֲ�Ĺ�ũ��������ũҩ������Һʱ����ʹ������Ͱ��ľͰ������ʹ����Ͱ����ԭ���ǣ��û�ѧ����ʽ��ʾ����
Fe+CuSO4=Cu+FeSO4
Fe+CuSO4=Cu+FeSO4
��
C���ɹ��ճɺ��Ϸ��β��溦����ʩ��Щ���������������ʣ�������������
���Ϸ�
���Ϸ�
������ʡ������ʡ������طʡ����Ϸʡ���
��6���������ִ������в���ȱ�ٵĴ������ߣ���ش��������⣺
A�����������������Ʒ�У��úϽ��������ǣ�����ĸ��
a
a
��
a���������b����������c������̥d����Ƥ����
B�����ڳ�ʪ�Ŀ�����������ʴ�������������ᣬ�����ӻ���������ʴ�������ԭ���Ǹ�����������������
ˮ
ˮ
��
C��β����ת�������ɽ�����β���е��ж�����ת��Ϊ�����壬��ͼ��ʾ��������β�����漰��Ӧ���۹��̣�
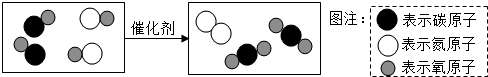
ͼ����ʾ��Ӧ�Ļ�ѧ����ʽΪ
��



 ���ɿ��õ�Ԫ����AB��ϵ�д�
���ɿ��õ�Ԫ����AB��ϵ�д� С�����ϵ�д�
С�����ϵ�д�
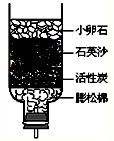 ��2009?��������ģ��Ŀǰȫ������������Դ��������ˮ��Դ����ʳ�ȷ����Σ����
��2009?��������ģ��Ŀǰȫ������������Դ��������ˮ��Դ����ʳ�ȷ����Σ����
 Ŀǰȫ������������Դ��������ˮ��Դ����ʳ�ȷ����Σ����
Ŀǰȫ������������Դ��������ˮ��Դ����ʳ�ȷ����Σ����