
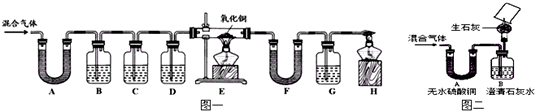


 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


| ʵ����� | ʵ������ | ʵ����� | |
| �� | �ڼ��촦��ȼ���壬 �ڻ����Ϸ���һ�� ������ྻ���ձ� |
�ձ��ڱ� ������ɫҺ�� |
�������������� �� |
| �� | �ڻ����Ϸ���һ��Ϳ ��������ʯ��ˮ���ձ� |
�ձ��ڳ��� ��ʯ��ˮ����� |
��������к���һ����̼ �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||

| ʵ����� | ���� | ���� |
ȡ�����Ĺ���̼�������Թ��У�����������ˮ���������ǵ�ľ�������Թܣ� ȡ�����Ĺ���̼�������Թ��У�����������ˮ���������ǵ�ľ�������Թܣ� |
�д������ݲ����������ǵ�ľ����ȼ �д������ݲ����������ǵ�ľ����ȼ |
����̼����������ˮ��Ѹ�ٷų����� ����̼����������ˮ��Ѹ�ٷų����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
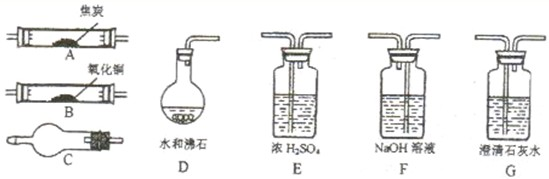
| ||
| ||
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com