

 ______________��
______________�� NaClO3+3H2��
NaClO3+3H2��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��θ�����IJ����ڿո�ʱ��ö��һЩ����֭ |
| B����pH��ֽ����ij��Һ�вⶨ��pH |
| C�����˲������뺬�д���������̼����У�����ȡ�κη�����ʩ�����¶����� |
| D�����ɻ���ʱ��Ϊ�������������������ʵ�ɬζ�ɼ�������ʳ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������������ȼ�ղ�������ɫ���棬������ɫ��ζ������ |
| B���ѽྻ��ͭ˿������������Һ�У���Һ����ɫ����ɫ |
| C����װ��Ũ�����Լ�ƿ��ƿ������ƿ���Ϸ��۲쵽���� |
| D���������ƹ��������粒�������ˮ����ų����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A��С�մ� |
| B����ºϽ� |
| C������� |
| D����ʯ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢۢ� | B���٢ڢۢ� | C���٢ۢܢ� | D���ڢۢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
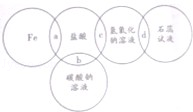
| A��a���γ�dz��ɫ��Һ | B��b���γɴ������� |
| C��c����Ӧ���� | D��d����Һ�ʺ�ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ������ | ��Һ�м��������� |
| ��һ�� | KCl��K2SO4��Na2CO3��NaCl |
| �ڶ��� | KCl��BaCl2��Na2SO4��K2CO3 |
| ������ | Na2SO4��KCl��K2CO3��NaCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
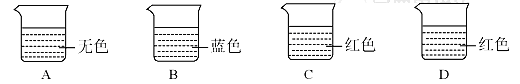
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com