������ʳƷ��������Ϊ����ϵ������������Ҫ�ɷ�Ϊ�������ۣ�
�����е�һ����Ҫ��ӦΪ��4Fe��OH��
2+O
2+2H
2O=4Fe��OH��
3 �����ɫ���壩
��1��Fe��OH��
2����
����������
����������
��
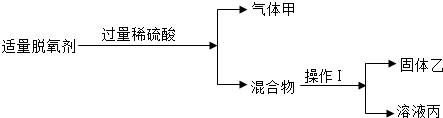
��2��ijͬѧ��ʳƷ��������2g���ۡ�0.5g����̿��0.5gʳ�Σ���������ʵ�飺
ʵ���
��ش�
��ͼ1���ٲ������������
����
����
��
����Һ���к��еĽ�����������
Fe2+��Na+
Fe2+��Na+
��д�����ţ�
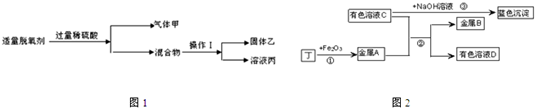
ʵ�����ϴ�Ӻ�ɺ�����̼ͨ�����ȵ������ɶ���������ͼ2ʵ�飬����֮���ת����ϵ��ͼ�����ֲ�����ȥ����
�ٽ���A��B�Ļ�ԣ�A
��
��
B���������������������
��������ɫ��ҺC����ɫ��ҺD�ķ�����
�۲���Һ��ɫ��dz��ɫΪFeSO4��Һ����ɫΪCuSO4��Һ
�۲���Һ��ɫ��dz��ɫΪFeSO4��Һ����ɫΪCuSO4��Һ
��
�۷�Ӧ�ٵĻ�ѧ����ʽΪ��
��
������ҺC�м���BaCl
2��Һ���а�ɫ�����������仯ѧ����ʽΪ
CuSO4+BaCl2=BaSO4��+CuCl2
CuSO4+BaCl2=BaSO4��+CuCl2
��




 �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д� ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�




 ��2012?ͨ������ģ�����������벻��������
��2012?ͨ������ģ�����������벻��������