
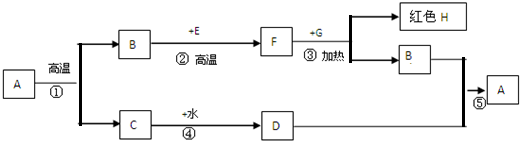
A�Ǽ����ǡ����ǡ�ʯ��ʯ����Ҫ�ɷ֣�B��F��Ԫ�������ͬ�����壬E��G�Ǻ�ɫ���壬�������й�ϵ�����ֲ�����ȥ����������и��⡣
|
|
|
|
|
|
|

|


|

|


|

|

|
|


|
|

|
��1��C�������� ��E�Ļ�ѧʽ ��
��2���۵Ļ�ѧ����ʽ�� ��
����F��һ���ʿ� ��������ʵ��ʱ�������װ���������⣬����ǰӦ ��Ŀ���� ��
��3���ܵĻ�ѧ����ʽ ��
��Ӧ�Ļ��������� ���÷�Ӧ�� ������ȡ����ȡ�����Ӧ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
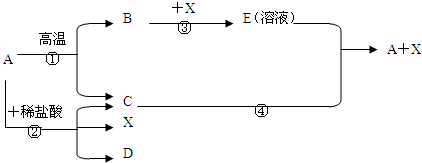
������A�Ǽ����ǵ���Ҫ�ɷ֣���һ���������ܷ�������ת�������³�ѹ�£�CΪ���壬XΪҺ�壬��C��X��Ϊ�����
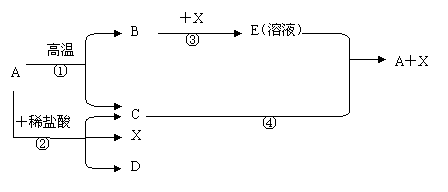
��ش��������⣺
��1��X�Ļ�ѧʽΪ����������������������
��2����Ӧ�ڵĻ�ѧ����ʽΪ��������������������������������
��3����Ӧ�ܵ�������������������������������������������
����Ӧ�Ļ�ѧ����ʽΪ����������������������������������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com