
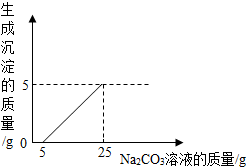
 ij��ѧС��ͬѧ��ʯ��ʯ�����ʲ����ᷴӦ��Ҳ������ˮ����ϡ���ᷴӦ��ȡ������̼���������÷�Һ�ⶨһƿNa2CO3��Һ�����ʵ��������������ǽ���Һ���ˣ�Ȼ������Һ�������μӸ�Na2CO3��Һ������Na2CO3��Һ�����������ɳ��������Ĺ�ϵ����ͼ��ʾ������Ӧ�Ļ�ѧ����ʽΪNa2CO3+CaCl2=CaCO3��+2NaCl������ش�
ij��ѧС��ͬѧ��ʯ��ʯ�����ʲ����ᷴӦ��Ҳ������ˮ����ϡ���ᷴӦ��ȡ������̼���������÷�Һ�ⶨһƿNa2CO3��Һ�����ʵ��������������ǽ���Һ���ˣ�Ȼ������Һ�������μӸ�Na2CO3��Һ������Na2CO3��Һ�����������ɳ��������Ĺ�ϵ����ͼ��ʾ������Ӧ�Ļ�ѧ����ʽΪNa2CO3+CaCl2=CaCO3��+2NaCl������ش�| 106 |
| x |
| 100 |
| 5g |
| ̼���Ƶ����� |
| ̼������Һ������ |
| 5.3g |
| 20g |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ�����н�����ѧ���꼶�п�ģ�⣨������ѧ�Ծ����������� ���ͣ�̽����
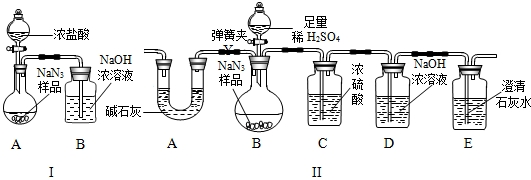
��10�֣��������ƣ�NaN3�����㷺Ӧ����������ȫ���ң�ij��ѧС��ͬѧ������������о���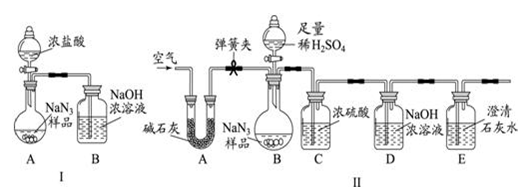
���������ϡ�
��NaN3��ײ��������Na��N2��
��NaN3�����ᡢH2SO4��Һ���������ɣ�
�ۼ�ʯ����CaO�� NaOH�Ļ����.
��NaN3���Ʊ������ǣ�����������Һ̬����Ӧ�Ƶ�NaNH2���ٽ�NaNH2��N2O��Ӧ������NaN3��NaOH�Ͱ�����NH3����
������̽����
��������ײ����30����������NaN3Ѹ�ٷֽ⣬д���÷�Ӧ�Ļ�ѧ����ʽ��
�� ��
�ƹ�ҵ��NaN3�г�����������Na2CO3����ԭ���ǣ��û�ѧ����ʽ��ʾ����
�� ��
��Ϊ�ⶨij��ҵ��NaN3��Ʒ�к���Na2CO3��������������ѧС��ͬѧ���������ʵ��
װ�á�
��С��ͬѧ��Ϊͨ���ⶨװ�� I�з�Ӧǰ��B���������ͨ����Ӧ���㣬�ͿɲⶨNa2CO3�Ĵ��ȣ�С����ͬѧͨ��������Ϊ�����ԣ������ɿ����� �� ��
��С��ͬѧ�ڴ�����۵Ļ����ϣ������װ��II���������װ��II��A������ ��������װ��C���Բⶨ�����ɵ�Ӱ���� �� ���ƫ����ƫС������Ӱ�족����
�۸���װ��II��С��ͬѧ��Ƶ�ʵ�鲽���У�
a������װ��D��
b��������Ʒ�����װ�������ԣ�
c�����ɼУ����������
d����Һ©���Ļ����Ͳ�������ע��������ϡ���ᣬ�رջ����Ͳ�������
����ȷ˳��Ϊ �� ������ĸ��ţ����ظ�����
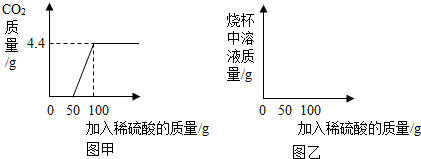
�ȸ�С���ijһ��ҵ��NaN3��Ʒ���м�⡣ȡ100�˸���Ʒ���ձ��У���ˮ�����ܽ⣬Ȼ����μ���һ����������������ϡ���Ტ������
���ȷ����ķ�Ӧ�ǣ�2Na2CO3+H2SO4=2NaHCO3+Na2SO4��Ȼ�����ķ�Ӧ�ǣ�2NaHCO3+H2SO4= Na2SO4+2H2O+2CO2�������������������ɶ�����̼�����������ϡ���������Ĺ�ϵ��ͼ����ʾ��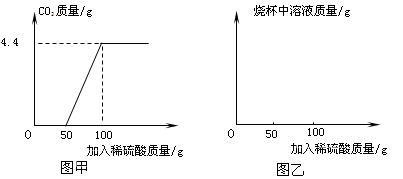
���������������Ϣ����ͼ�ҵ�����ϵ�л������������ձ�����Һ��������μ�������Һ�����ı仯���ߡ�
�ڼ������Ʒ�д��������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�챱���д������п�һģ��ѧ�Ծ����������� ���ͣ�̽����
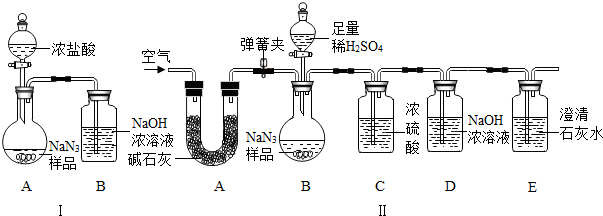
��8�֣���������(NaN3�����㷺Ӧ����������ȫ���ң�ij��ѧС��ͬѧ������������о���
���������ϡ�
��NaN3��ײ��������Na��N2��
��NaN3�����ᡢH2SO4��Һ���������ɡ�
�ۼ�ʯ����CaO�� NaOH�Ļ���
��NaN3���Ʊ������ǣ�����������Һ̬����Ӧ�Ƶ�NaNH2���ٽ�NaNH2��N2O��Ӧ������NaN3��NaOH�Ͱ�����NH3����
������̽����
��1��������ײ����30����������NaN3Ѹ�ٷֽ⣬д���÷�Ӧ�Ļ�ѧ����ʽ ��
��2����ҵ��NaN3�г�����������Na2CO3����ԭ���ǣ��û�ѧ����ʽ��ʾ�� ��
��3��Ϊ�ⶨij��ҵ��NaN3��Ʒ�к���Na2CO3��������������ѧС��ͬѧ���������ʵ��װ�á�
��С��ͬѧ��Ϊͨ���ⶨװ�â��з�Ӧǰ��B���������ͨ����Ӧ���㣬�ͿɲⶨNa2CO3�Ĵ��ȣ�С����ͬѧͨ��������Ϊ�����ԣ������ɿ����� �����
��С��ͬѧ�ڴ�����۵Ļ����ϣ������װ�â��������װ�â���A��������_____������װ��C���Բⶨ�����ɵ�Ӱ���� ���ƫ����ƫС������Ӱ�족����װ��E�������� ��
�۸���װ�â�С��ͬѧ��Ƶ�ʵ�鲽���У�a������װ��D��b.������Ʒ�����װ�������ԡ�c.���ɼУ����������d.��Һ©���Ļ����Ͳ�������ע��������ϡ���ᣬ�رջ����Ͳ�����������ȷ˳��Ϊ��������������ĸ��ţ����ظ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�걱���д������п���ѧһģ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com