
ijͬѧΪ�ⶨ10gij������ʯ������������������������ϡ�����п����ȡ�������������ͼ��ʾ��װ�ã������йص�ʵ��̽������ʾ��3H2+Fe2O3 2Fe+3H2O�����ʲ��μӷ�Ӧ���ٶ�ÿ������ȫ��Ӧ�����գ�����ش��й����⣺
2Fe+3H2O�����ʲ��μӷ�Ӧ���ٶ�ÿ������ȫ��Ӧ�����գ�����ش��й����⣺
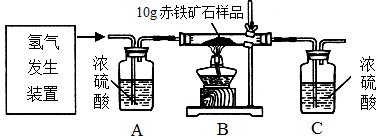
�õ��������ݣ�
| װ�� | B | C |
| ��Ӧǰ���� | 84.3g | 294.1g |
| ��Ӧ������ | 81.9g | 296.8g |
��1�������ó�����ʯ��Ʒ����������������������д��������̣�
��2����ʵ�黹�ɲⶨ���ˮ�и�Ԫ��֮���������ϵ�����ñ���ʵ��������ʽ��ʾ��ˮ���⡢��Ԫ�ص�������Ϊ_______________________________________________��ֻ��ʽ�������㣩�����������е�������û����ȫ��Ӧ�������ˮ���⡢��Ԫ�ص������Ȼ�__________��ѡ�ƫ����ƫС�� ���䡱����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�꽭��ʡ�Ͼ��й�¥���п���ģ��ѧ�Ծ����������� ���ͣ�������
ijͬѧΪ�ⶨ10gij������ʯ������������������������ϡ�����п����ȡ�������������ͼ��ʾ��װ�ã������йص�ʵ��̽������ʾ��3H2+Fe2O3 2Fe+3H2O�����ʲ��μӷ�Ӧ���ٶ�ÿ������ȫ��Ӧ�����գ�����ش��й����⣺
2Fe+3H2O�����ʲ��μӷ�Ӧ���ٶ�ÿ������ȫ��Ӧ�����գ�����ش��й����⣺
�õ��������ݣ�
| װ�� | B | C |
| ��Ӧǰ���� | 84.3g | 294.1g |
| ��Ӧ������ | 81.9g | 296.8g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�꽭��ʡ�Ͼ��й�¥���п���ģ��ѧ�Ծ��������棩 ���ͣ�������
ijͬѧΪ�ⶨ10gij������ʯ������������������������ϡ�����п����ȡ�������������ͼ��ʾ��װ�ã������йص�ʵ��̽������ʾ��3H2+Fe2O3 2Fe+3H2O�����ʲ��μӷ�Ӧ���ٶ�ÿ������ȫ��Ӧ�����գ�����ش��й����⣺
2Fe+3H2O�����ʲ��μӷ�Ӧ���ٶ�ÿ������ȫ��Ӧ�����գ�����ش��й����⣺

�õ��������ݣ�
|
װ�� |
B |
C |
|
��Ӧǰ���� |
84.3g |
294.1g |
|
��Ӧ������ |
81.9g |
296.8g |
��1�������ó�����ʯ��Ʒ����������������������д��������̣�
��2����ʵ�黹�ɲⶨ���ˮ�и�Ԫ��֮���������ϵ�����ñ���ʵ��������ʽ��ʾ��ˮ���⡢��Ԫ�ص�������Ϊ_______________________________________________��ֻ��ʽ�������㣩�����������е�������û����ȫ��Ӧ�������ˮ���⡢��Ԫ�ص������Ȼ�__________��ѡ�ƫ����ƫС�� ���䡱����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com