


 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
| ʵ����� | 1 | 2 | 3 |
| ϡ����������g�� | 50 | 50 | 100 |
| ��Ʒ������g�� | 20 | 30 | 20 |
| ���������������g�� | 6.6 | 6.6 | 6.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
 2CuO + H2O +
2CuO + H2O + ������
������  ���Ļ�ѧʽ�� ��
���Ļ�ѧʽ�� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
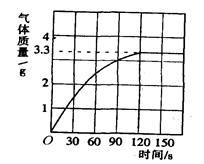
 CaO + CO2 �� ����÷�Ӧ����������(m)�뷴Ӧʱ��(t)�Ĺ�ϵ���±���
CaO + CO2 �� ����÷�Ӧ����������(m)�뷴Ӧʱ��(t)�Ĺ�ϵ���±���| ��Ӧʱ��t�Ms | t0 | t1 | t2 | t3 | t4 | t5 | t6 |
| ��Ӧ����������m�Mg | 100 | 92 | 84 | 78 | 72 | 67 | 67 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
| | ��һ�� | �ڶ��� | ������ |
| ��ȡ�Ͻ������/g | 25 | 25 | 50 |
| ����ϡ���������/g | 120 | 160 | 100 |
| ��ַ�Ӧ��ʣ������֮��/g | 144.6 | 184.6 | 149.6 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com