��2012?ͨ����һģ����ѧ������ϢϢ��أ����ǵ���ʳס�ж��벻����ѧ��
��1��������������������ȷ����
BD
BD
��
A�������յķ���������ë�͵��� B���û���̿ʹӲˮ����
C���ù۲�ķ���������ۺ����� D���ø�˿���ϴ����
��2��Ŀǰһ�ֽе����[Ca��IO
3��
2]�ı���Ʒ�ѽ���һЩ���У����к�������������Ԫ����
��
��
��Ca��IO
3��
2��I�Ļ��ϼ�Ϊ
+5
+5
��
��3����ʳ��ƿ�ǣ����ŵ�һ����ζ��˵�����Ӿ��е�������
�����Dz����˶���
�����Dz����˶���
����pH��ֽ�ⶨʳ����ȵIJ�����
�ڲ���Ƭ�Ϸ�һСƬpH��ֽ����������Һ�ε���ֽ�ϣ�����ʾ����ɫ���ɫ���Ƚ�
�ڲ���Ƭ�Ϸ�һСƬpH��ֽ����������Һ�ε���ֽ�ϣ�����ʾ����ɫ���ɫ���Ƚ�
��
��4�������Ҵ������ǽ��Ҵ���C
2H
5OH�������Ͱ�һ��������϶��ɵ�һ������ȼ�ϣ��Ҵ������;�����
���
���
���������л�������Ҵ�������̼Ԫ������Ԫ�ص�������Ϊ
4��1
4��1
����100t����������
11.1
11.1
t�Ҵ������Ƶõ����Ҵ���������Ϊ10%�ij����Ҵ����ͣ���ȷ��0.1����




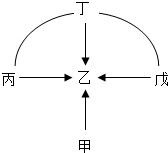 ��2012?ͨ����һģ��30���ס��ҡ������������dz��л�ѧ���������ʣ������Ӧ��ϵ��ͼ����������ʾת����ϵ����-����ʾ���Ӧ��ϵ���������������ش����⣺
��2012?ͨ����һģ��30���ס��ҡ������������dz��л�ѧ���������ʣ������Ӧ��ϵ��ͼ����������ʾת����ϵ����-����ʾ���Ӧ��ϵ���������������ش����⣺