
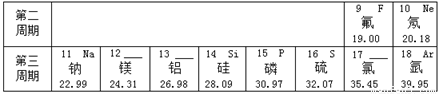
��Ԫ�����ڱ���ָ���£�����Ԫ��֮���һЩ������֪ʶ������ѧϰ���ʵ����ʣ���ʹ��ѧѧϰ���о�����й��ɿ�ѭ����ͼ��ʾ��Ԫ�����ڱ���һ���֡�
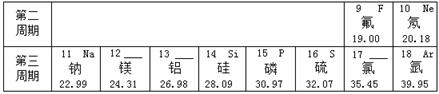
��1���ؿ��к������Ľ���Ԫ���� ���� ��дԪ�ط��ţ�����������Ϊ ��
��2���ؿ��й�Ԫ�صĺ�����������Ԫ�أ���Ԫ�ص����ԭ������Ϊ ��
��3�����������о�������ȶ��ṹ��ԭ�ӵ�ԭ������Ϊ ��
��4�� ��11��Ԫ����17��Ԫ����ɻ�����ʱ�����ɴ����ʵ������ӵķ���Ϊ ��
��5����ͼ��ʾ�����У�����ͬ��Ԫ�ص��� ������ţ���Ԫ�صĻ�ѧ�������Ƶ��� ������ţ�����A��D����Ԫ����ɵ����ʵĻ�ѧʽΪ ��
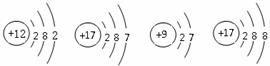
A B C D.
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ������Ϊ������Ľ������������绯ѧʷ���أ�����������1825��ű�Ӣ����ѧ�Ҵ�ά�Ƶã����죬���Ѿ����������������ÿһ�����䣮
������Ϊ������Ľ������������绯ѧʷ���أ�����������1825��ű�Ӣ����ѧ�Ҵ�ά�Ƶã����죬���Ѿ����������������ÿһ�����䣮
| ||
| ||
| ||
| ||
| ʵ�鷽�� | ʵ������ | ���� |
| ��ȡһ�����ĺϽ��ĩ���ӹ�����30%NaOH��Һ����ַ�Ӧ����ˣ��������ã� | ��ĩ�����ܽ⣬��������ų��� | �Ͻ���һ������ �� �� �� |
| ��ȡ����������������ӹ����� 10%���� 10%���� ����ַ�Ӧ�� |
���������ܽ⣬��������ų�����Һ��dz��ɫ�� | �Ͻ���һ������ ����ͭ ����ͭ �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��㶫ʡ�����лݳ������꼶��ѧ�ڵڶ���������ѧ�Ծ��������棩 ���ͣ������
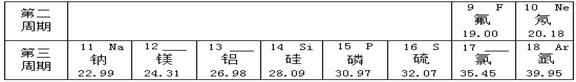
��Ԫ�����ڱ���ָ���£�����Ԫ��֮���һЩ������֪ʶ������ѧϰ���ʵ����ʣ���ʹ��ѧѧϰ���о�����й��ɿ�ѭ����ͼ��ʾ��Ԫ�����ڱ���һ���֡�

��1���ؿ��к������Ľ���Ԫ���� ��дԪ�ط��ţ�����������Ϊ ��
��2���ؿ��й�Ԫ�صĺ�����������Ԫ�أ���Ԫ�ص����ԭ������Ϊ ��
��3�����������о�������ȶ��ṹ��ԭ�ӵ�ԭ������Ϊ ��
��4����11��Ԫ����17��Ԫ����ɻ�����ʱ�����ɴ����ʵ������ӵķ���Ϊ ��
��5����ͼ��ʾ�����У�����ͬ��Ԫ�ص��� ������ţ���Ԫ�صĻ�ѧ�������Ƶ��� ������ţ�����A��D����Ԫ����ɵ����ʵĻ�ѧʽΪ ��

A B C D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�걱���к�����������ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
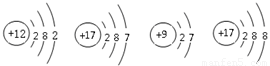
��Ԫ�����ڱ���ָ���£�����Ԫ��֮���һЩ������֪ʶ������ѧϰ���ʵ����ʣ���ʹ��ѧѧϰ���о�����й��ɿ�ѭ����ͼ��ʾ��Ԫ�����ڱ���һ���֡�
��1���ؿ��к������Ľ���Ԫ���� ���� ��дԪ�ط��ţ�����������Ϊ ��
��2���ؿ��й�Ԫ�صĺ�����������Ԫ�أ���Ԫ�ص����ԭ������Ϊ ��
��3�����������о�������ȶ��ṹ��ԭ�ӵ�ԭ������Ϊ ��
��4�� ��11��Ԫ����17��Ԫ����ɻ�����ʱ�����ɴ����ʵ������ӵķ���Ϊ ��
��5����ͼ��ʾ�����У�����ͬ��Ԫ�ص��� ������ţ���Ԫ�صĻ�ѧ�������Ƶ���
������ţ�����A��D����Ԫ����ɵ����ʵĻ�ѧʽΪ ��
A B C D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ԫ�����ڱ���ָ���£�����Ԫ��֮���һЩ������֪ʶ������ѧϰ���ʵ����ʣ���ʹ��ѧѧϰ���о�����й��ɿ�ѭ����ͼ��ʾ��Ԫ�����ڱ���һ���֡�
��1���ؿ��к������Ľ���Ԫ���� ���� ��дԪ�ط��ţ�����������Ϊ ��
��2���ؿ��й�Ԫ�صĺ�����������Ԫ�أ���Ԫ�ص����ԭ������Ϊ ��
��3�����������о�������ȶ��ṹ��ԭ�ӵ�ԭ������Ϊ ��
��4����11��Ԫ����17��Ԫ����ɻ�����ʱ�����ɴ����ʵ������ӵķ���Ϊ ��
��5����ͼ��ʾ�����У�����ͬ��Ԫ�ص��� ������ţ���Ԫ�صĻ�ѧ�������Ƶ��� ������ţ�����A��D����Ԫ����ɵ����ʵĻ�ѧʽΪ ��
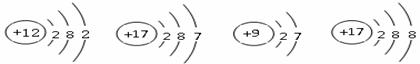
A B C D
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com