
(9��) һ��Ȥζ��ѧ��У�����ʦ��ͬѧ��չʾ��һƿ��ǩ�������ɫ��Һ����ͼ��ʾ��Ҫ��ͬѧ�ǽ���̽����ȷ����ƿ��Һ������ʲô��Һ��
��������롿 ����ʦ��ʾ����ƿ��ɫ��Һֻ��������������Һ�е�һ�֣�������þ��Һ ����������Һ ��������Һ���������Һ
���������ϡ�
�ٳ����£�������ʵ��ܽ�����£�
| ���� | MgSO4 | Na2SO4 | (NH4)2SO4 | H2SO4 |
| �ܽ�� | 35��1g | 19��5g | 75��4g | ��ˮ����Ȼ��� |
| ʵ����� | ʵ������ | ʵ����� |
| ��ȡ����Һ�������Թ��У������еμӼ��� ��Һ | ��Һ���а�ɫ�������� | ����ٳ��� |
| ���ò�����պȡ����ԭ��Һ����pH��ֽ�ϣ���������ɫ������ | ��ҺpHС��7 | ����۳��� |
| ʵ����� | ʵ������ | ʵ����� |
| ȡ����Һ�������Թ��У� | | ����ܳ������÷�Ӧ�Ļ�ѧ����ʽΪ |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ͼ�ش����⣺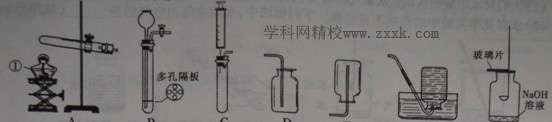
(1)д��ͼ�б���������ƣ��� ��
��2��ʵ�����ø��������ȡ���ռ�����Ӧѡ�õ�װ���� ������ţ���ͬ������Ӧ�Ļ�ѧ����ʽΪ �����װ��������ʱ���������ֽ��յķ����⣬�����Բ��õķ�����
(3)ʵ���ҿ��������ƹ�����Ũ��ˮ�ڳ����»����ȡ����������Ϊ�˵õ�ƽ�ȵİ�������Ӧѡ��ķ���װ���� ��
(4)��Gװ��������������ȼ��ʵ�飬ȼ�ս�����ȡ��ȼ�ճף������ò���Ƭ��סƿ�ڲ�����������ƿ��ת�����ֲ���Ƭ�� ����ס������������(���ͼ)����ԭ���� ��
(5)Ϊ�������ʵ�飬��lOmL��Ͳ��ȡ6mL����������Һ������ʱ��Ͳ�ڵ�Һ�尼Һ����ʹ�Ӧ����ͼ�� ��(�a"��b��)�̶��߱���ˮƽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�̽����
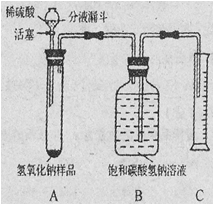
(10��)��ѧ����������̫���ܼ���������CO2�������ͷ�CO2����ʵ��̼ѭ��(��ͼ��ʾ)��
ij��ѧС���ͬѧ�Դ˷dz�����Ȥ������ʦ��ָ���£��������װ��̽����������ķ�Ӧԭ���Ƿ���С�
��1����֤��װ��A���ͷ�CO2���������� ��
��2��װ��B��ʵ����������ƾ����ʱ�������� ��
��3��������Ӧ������С��ͬѧ��D�й���ijɷֽ���̽������֤���Ƿ�����CO2��
����������衿
D�еĹ������Ϊ��I��ֻ�������ƣ�����������̼��ƣ���ֻ��̼���
������ʵ�顿
�ټ�ͬѧ��D��ȡһ�����Ĺ������Թ��У�������һ������ˮ�����а�ɫ�������ͬѧ�ݴ���Ϊ�Թ��еĹ���Ϊ̼��ƣ���������������ͬѧ��Ϊ����ʵ�鲻����֤�������������������� ��
����ͬѧ��D��ȡһ�����Ĺ������Թ��У�����һ������ˮ�������Թ���ڣ��о����ȣ��������Թ��м��뼸��ϡ���ᣬû�з������ݲ�������ͬѧ�ݴ���Ϊ�Թ��еĹ���ֻ�������ƣ�������I��������ͬѧ��Ϊ�ҵ�ʵ�鲻����֤������I������������ ��
�۱�ͬѧ��Ϊ���øо��¶ȱ仯�ķ����ж��Ƿ��������Ʋ��ɿ�������ˮ����̪��Һ��
ϡ����֤���˲������������������ʵ�鱨�档
| ʵ�鲽�� | ʵ������ | ʵ����� |
| | | �������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�̽����
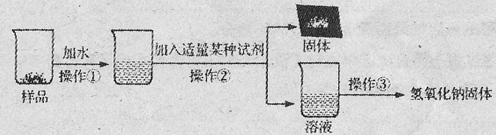
��15�֣�ʵ������һƿ���ڱ�¶�ڿ����е��������ƹ�����Ʒ���۲췢�֣���Ʒ�����а�ɫ��ĩ��ij��ȤС���ͬѧ�Ը���Ʒ�ijɷּ�����������̽����
��������⡿����Ʒ�к�����Щ���ʣ�
��������롿ͨ��������������²��룺
���������ȫ���ʣ�����Ʒ��ֻ��Na2CO3��
������ֱ��ʣ�����Ʒ�к���NaOH��Na2CO3��
��NaOH���ʷ�Ӧ�Ļ�ѧ����ʽΪ ��
���������ϡ�
�ټ��Ե�Na2CO3��Һ���������Ե�CaCl2��Һ�������ֽⷴӦ��
��CO2�ڱ���̼��������Һ�м������ܽ⡣
��ʵ��̽��1��Ϊȷ������Ʒ�ijɷ֣�С�����������ʵ�鷽��������һ���������ʵ�鱨�档
| ʵ����� | ʵ������ | ʵ����� |
| ��1��ȡ������Ʒ����ˮ������ �� | ��ɫ�������� | �÷�Ӧ�Ļ�ѧ����ʽ�� �� |
| ��2����������Ӧ��Ļ������ˣ�ȡ��Һ���� �� | �� | ֤���������ȷ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�̽����
��7�֣�ij�о���ѧϰС������֤����ʹ��̪��Һ��족��ʵ��ʱ������һ������������̪��Һ����ijNaOH��Һ�У���Һ����˺�ɫ�����ǹ�һ�����ɫȴ��ʧ�ˡ�
��������⡿��ʲôԭ���µ��з�̪��NaOH��Һ�ɺ�ɫ��Ϊ��ɫ��
����������衿���Ƿֱ�������������������²��룺
��ͬѧ�������Ƿ�̪��O2�����˷�Ӧ��
��ͬѧ��������NaOH��Һ������е�CO2�����˷�Ӧ��
��ͬѧ��������NaOH��ҺŨ�ȴ�С�йأ�
����Ϊ���ܻ���_________________________________�йأ�дһ�����ɣ���
�����ʵ�顿��λͬѧ�ֱ����ʵ����֤�Լ��IJ��룺
��1����ͬѧ���������ʵ�飬������д�±���
| ʵ�鲽�� | �����һ�����Ŀ�� | ʵ������ | ʵ����� |
| 1����NaOH��Һ������� | | ��Һ��죬��һ�����ɫ��ʧ | ��ͬѧ����_______�����ȷ������ȷ���� |
| 2������ȴ�����Һ�е��˷�̪������һЩֲ���������Ϸ� | |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�̽����
��ͼ��ʾ��ʵ������һƿ��ǩ�����ָ�ʴ����Һ����֪����Һ����NaNO3��Ϊȷ����ɷ֣�ijͬѧ���������о���
�������жϡ���1������Һ������������������ţ�������ڼ���Σ�
��ʵ��̽������λͬѧ���������ʵ��
��������������
��2��������һ���������Բ������ô������� ����
��3����ɡ�����һ��������������Һһ������ ��Һ��д���ʻ�ѧʽ������������� ��
��4��������ơ�����������
�����X�Լ��� ����ʵ�������� ��������������Һ�� ��д���ʻ�ѧʽ����
����˼�����ۡ�
��5������ʵ���У����� ����д��ţ���û�б�Ҫ���еġ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�̽����
(4��)С��Ϊ̽��п������ͭ���ֽ����Ļ��˳�����������ͼ��ʾ������ʵ�飺
��ʵ��һ�У�С���ԡ���������������ݵĿ�����Ϊ�����ж�п�����Ļ��ǿ������ͬѧ��Ϊ���������������� ��
��ʵ����У������� ����֤�����Ļ��ǿ��ͭ���÷�Ӧ�Ļ�ѧ����ʽΪ___________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�̽����
��11�֣�ijʵ��С���ͬѧҪ̽���������̼���ƵĻ�ѧ���ʣ����������ʵ��װ�ü�������
| ʵ��װ�� | ʵ�鲽�輰���� | ʵ������ |
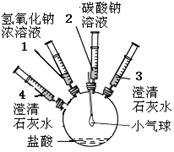 | �ٽ�ע����2�е���Һ����ʢ��ϡ�����ƿ�У������������ݲ����� | ������ |
| �ڽ�ע����3���������� | ����ʯ��ˮ����� | |
| �۽�ע����1�е���Һ����ƿ�� | ������� | |
| �� | ���������� | |
| �ݽ�ע����4�е���Һ����ƿ�� | |
| ʵ����� | ʵ������ | ʵ����� |
| �ֱ�ȡ������Һ��A��B��֧�Թ��У�A�� ����CaCl2��Һ��B�м���Na2CO3��Һ | A�в�����ɫ������B��û�г��� | ƿ����Һ�����ʵijɷ�Ϊ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�̽����
��7�֣���ʦ��ʵ���Ҵ�����7ƿ��ͬ����ɫ��Һ������ͼ��ʾ��������3��7���Լ�ƿ��ǩ��ȫ����4��5���Լ�ƿ��ǩ�������𡣸��ݲ���ҩƷ��¼����֪��3��4��5��7����Һ�ֱ���NaOH��Һ��Na2CO3��Һ��ʯ��ˮ��CaCl2��Һ�е�һ�֡�����ʦ��ָ���£���ѧС��ͬѧ����������ʵ�顣
����֪�� Na2CO3��Һ��CaCl2��Һ�ܷ������ֽⷴӦ��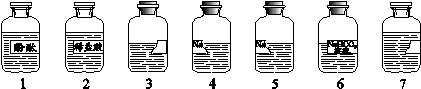
��1���ֱ�ȡ3��4��5��7����Һ��4֧�Թ��У������зֱ�μ�1����Һ������3��4��5����Һ��죬˵����3����Һ�� ������ԡ��������ԡ������ԡ������ɸ�ʵ���ȷ������ҺΪ ��д���Լ�ƿ��ż���Ӧ����Һ���ƣ���
��2���ֱ�ȡ4�š�5����Һ����֧�Թ��У�����������2����Һ�����־������ݲ�����
С��ͬѧ���������Լ�����������ʵ������ȷ����������Һ�ijɷ֡�
| ʵ����������� | ʵ����� |
| | 4����Һ��Na2CO3��Һ��5����Һ�DZ��ʵ�NaOH��Һ�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com