
���������������ƿ��У��γ������ܣ�������������һС�ڽ����ƣ���ͼ1�����Ѵ�����Ƥ���IJ����ܲ���ʢ��dz��ɫ��������ƿ�У���ͼ2����ƿ�ײ�����������ϸɳ�����������е��뼸��ˮ�����������ܵ��ƿ��Һ�����£���ͼ3������ƿ���Ȳ����������̣��̶�ȼ�գ�dz��ɫ����ʧ����ͼ4�������գ���ɫ������������ƿ����ͼ5����
������ʵ���ƶϣ������Ƶ�Ӳ��________���۵�________��ˮ����������Ʒ�Ӧ_______������ա��ų�������������ƿ�г����İ�ɫ����������__________��
 �����ܿ����ϵ�д�
�����ܿ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ӷ��ӡ�ԭ�ӵĽǶ���ʶ��ѧ��Ӧ�ǻ�ѧ�о��Ļ�����������ͼ��ij�ܱ����������ʱ仯���̵���ʾ��ͼ������ ����ʾ��ԭ�ӣ���
����ʾ��ԭ�ӣ��� ����ʾ��ԭ�ӣ�
����ʾ��ԭ�ӣ�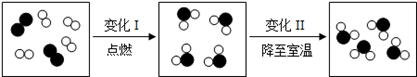
��ش�
��1���仯��Ļ�ѧ����ʽ���� ��
��2�����й��ڱ仯���˵���У���ȷ������ ������д��ţ���
| A�����ӵ���Ŀ������ | B�����ӱ�С�� |
| C�����Ӽ�ľ����С�� | D�����ӵ�������˸ı� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����������ʵ����ʣ�д����Ӧ��һ����;��
��1�����ʯ��Ӳ�ȴ� ��
��2���ɱ��������� ��
��3�������µ����Ļ�ѧ���ʲ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѡ����Ӧ���ʵ������գ�
�ٸɱ� ��С�մ� ��ʯī ���Ȼ���
��1����������Ǧ��о������ ����
��2���������˹���������� ����
��3��ҽ������������������ˮ������ ����
��4�����Ƹ�����÷��ͷ۵���Ҫ�ɷ����� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ʵ����ʾ������ʵ���;�������������ʵĻ�ѧʽ��գ�
| A���ɱ� | B��һ����̼ | C�������� | D������ E���������� F��̼���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
2013���й�������Э��Բ���װ����խ�ڻ���״���������Է��֣����������ڱ���Ⱦ������һ��û����ɫ����������ζҺ�壮�ܶȱ�ˮС��������ˮ�����ķе���80.1�棬�۵���5.5�森��һ�������£����ܸ��������塢Ũ�����Ũ��������ʷ�����ѧ��Ӧ���������ڿ�����ȼ�����ɶ�����̼��ˮ����ش��������⣺
��1������һ���������� ��
��2������һ����ѧ������ ��
��3��д�����ڿ�����ȼ�յ����ֱ���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
Ŀǰ���ҹ���涨�ڹ�������ȫ����̡�������ʾ��Լ12%�й����Ե������������̲�ʹ�ã�ÿ����60����������̲�������¶���������̲ݵ���Ҫ�ɷ��̼������Ŷ�(C10H14N2)��ʹ��������������Բ��������ּ����������й��̼��˵���������
| A���̼������л��� | B���̼���̼����Ԫ��������Ϊ12:1 |
| C���̼����Է�������Ϊ162 | D���̼���̼Ԫ�ص������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���мӵ�Ԫ�صĻ��ϼ��ɵ͵��ߵ�����˳��������ǣ�������
��N2 ��NO2 ��HNO3 ��NH3��
| A���٢ڢۢ� | B���ܢ٢ڢ� | C���٢ۢڢ� | D���٢ܢڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����������CrO3�������ڽ����Ƹ�����ҵ���������������Ļ�ѧ����ʽΪ��
X��H2SO4==== 2CrO3��Na2SO4��H2O������X�Ļ�ѧʽ��
| A��Na2CrO4 | B��Na2Cr2O7 | C��Na2Cr2O4 | D��Na2CrO7 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com