
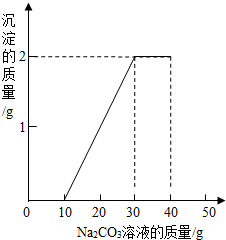
 ij��ͬѧ�����ȡ������̼��ʵ��Ի��յ�������Ȼ��ƻ��Һ���������������ʣ���������ʵ�飺ȡһ�����û��Һ���ձ��У��μ�40g������������Ϊ10.6%��̼������Һ�Ƶ��ձ�����Һ������Ϊ68.5g������̼������Һ�����������ɳ���������ϵ��ͼ��ʾ��
ij��ͬѧ�����ȡ������̼��ʵ��Ի��յ�������Ȼ��ƻ��Һ���������������ʣ���������ʵ�飺ȡһ�����û��Һ���ձ��У��μ�40g������������Ϊ10.6%��̼������Һ�Ƶ��ձ�����Һ������Ϊ68.5g������̼������Һ�����������ɳ���������ϵ��ͼ��ʾ��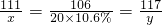
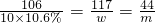
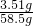 ��100%=6%
��100%=6%

 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
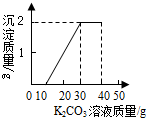 ij��ͬѧ�����ȡ������̼��ʵ��Ի��յ�������Ȼ��ƻ����Һ���������������ʣ�����������ʵ�飺ȡ40mL����Һ���ձ��У�����40g������������Ϊ13.8%��K2CO3��Һ������K2CO3��Һ���������ɳ��������Ĺ�ϵ��ͼ��ʾ����
ij��ͬѧ�����ȡ������̼��ʵ��Ի��յ�������Ȼ��ƻ����Һ���������������ʣ�����������ʵ�飺ȡ40mL����Һ���ձ��У�����40g������������Ϊ13.8%��K2CO3��Һ������K2CO3��Һ���������ɳ��������Ĺ�ϵ��ͼ��ʾ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
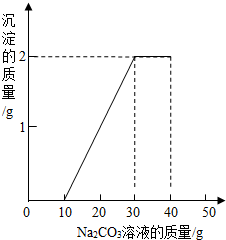 ij��ͬѧ�����ȡ������̼��ʵ��Ի��յ�������Ȼ��ƻ��Һ���������������ʣ���������ʵ�飺ȡһ�����û��Һ���ձ��У��μ�40g������������Ϊ10.6%��̼������Һ�Ƶ��ձ�����Һ������Ϊ68.5g������̼������Һ�����������ɳ���������ϵ��ͼ��ʾ��
ij��ͬѧ�����ȡ������̼��ʵ��Ի��յ�������Ȼ��ƻ��Һ���������������ʣ���������ʵ�飺ȡһ�����û��Һ���ձ��У��μ�40g������������Ϊ10.6%��̼������Һ�Ƶ��ձ�����Һ������Ϊ68.5g������̼������Һ�����������ɳ���������ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
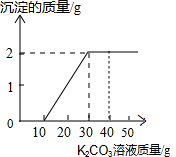 ij��ͬѧ�����ȡ������̼��ʵ��Ի��յ�������Ȼ��ƻ����Һ���������������ʣ�����������ʵ�飺ȡ40g����Һ���ձ��У�����40g������������Ϊ 13.8%��K2CO3��Һ������K2CO3��Һ���������ɳ��������Ĺ�ϵ����ͼ��ʾ���ش��������⣺�����������1λС����
ij��ͬѧ�����ȡ������̼��ʵ��Ի��յ�������Ȼ��ƻ����Һ���������������ʣ�����������ʵ�飺ȡ40g����Һ���ձ��У�����40g������������Ϊ 13.8%��K2CO3��Һ������K2CO3��Һ���������ɳ��������Ĺ�ϵ����ͼ��ʾ���ش��������⣺�����������1λС�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
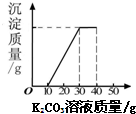 ij��ͬѧ�����ȡ������̼��ʵ��Ի��յ�������Ȼ��ƻ����Һ���������������ʣ�����������ʵ�飺ȡ40mL����Һ���ձ��У�����40g������������Ϊ13.8%��K2CO3��Һ������K2CO3��Һ���������ɳ��������Ĺ�ϵ����ͼ��ʾ����
ij��ͬѧ�����ȡ������̼��ʵ��Ի��յ�������Ȼ��ƻ����Һ���������������ʣ�����������ʵ�飺ȡ40mL����Һ���ձ��У�����40g������������Ϊ13.8%��K2CO3��Һ������K2CO3��Һ���������ɳ��������Ĺ�ϵ����ͼ��ʾ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�꽭��ʡ���о��ݶ�����ǰ�������Ի�ѧģ���Ծ��������棩 ���ͣ������
 ��2006?������ij��ͬѧ�����ȡ������̼��ʵ��Ի��յ�������Ȼ��ƻ����Һ���������������ʣ�����������ʵ�飺ȡ40mL����Һ���ձ��У�����40g������������Ϊ13.8%��K2CO3��Һ������K2CO3��Һ���������ɳ��������Ĺ�ϵ��ͼ��ʾ����
��2006?������ij��ͬѧ�����ȡ������̼��ʵ��Ի��յ�������Ȼ��ƻ����Һ���������������ʣ�����������ʵ�飺ȡ40mL����Һ���ձ��У�����40g������������Ϊ13.8%��K2CO3��Һ������K2CO3��Һ���������ɳ��������Ĺ�ϵ��ͼ��ʾ�����鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com