
| �к��Լ� | �������� | �������� |
| �۸�/��Ԫ?��-1�� | 800 | 450 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��Լ��Դ�ͱ��������Ѿ���Ϊ���ǵĻ������ߡ����ܼ��š�������������̬�н�������������Щ��Ĺ����ص㡣���᳧��������������ǣ��Ѻ������ȼ�գ����ɶ�������������������ڸ��ºʹ���������������������������������ˮ�����������ᡣд�����������ˮ������������Ļ�ѧ����ʽ
ij������Ʒ�к������������ƣ������ⶨ��̼���Ƶ��������������ú�����������ij����ˮ��������ʵ�飺
��ʵ��ԭ����Na2CO3+H2SO4= Na2SO4 + H2O + CO2��ͨ��ʵ��ⶨ��Ӧ�����Ķ�����̼���������������ԭ��Ʒ��̼���Ƶ�����,�������̼��������Ʒ�е�����������
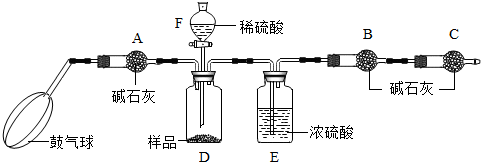
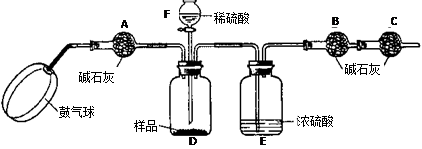
��ʵ��װ�á�
��ʵ�鲽�衷
����ͼ����װ�ã���B��C�⣩����������ҩƷ��
�ڳ�������¼B��������m1����������ʱע����B�����ˡ���
�۰������������������Լ1���ӡ�
��������B��C��
�ݴ�Һ©��F�Ļ�������ϡ������ټ���D�кرջ�����
�ް�����������Լ1���ӡ�
�߳�������¼B��������m2����������ʱע����B�����˼�E�Ҷ˵ij��ڡ���
����㡣
����֪��ʯ�ҵ���Ҫ�ɷ����������ƺ��������ƣ�������A�������ǣ� ������ʹ�ⶨ���ƫ��
��2�� ���ܻ��ܣ���ϡ�������ϡ���ᣬ��Ϊ������� �ԣ���ʹ���̼���Ƶ��������� ����ƫ��ƫС�䣬��ͬ������ȥ�������C������̼���Ƶ�������������
��3��Eװ�õ�������
��4����ʵ���ܷ�ʡ�Ԣۡ�����������? �����ܻ��ܣ���ԭ��ֱ��� ��
��5������ȡ��Ʒ������Ϊ6g����Һ©��F��ʢ��5��ֻ������һ�����ʵij����ˮ���Ƶ�m1Ϊ51.20g��m2Ϊ53.40g����������������λС����
��1����Ʒ��̼���Ƶ���������Ϊ���٣�
��2��Dװ�������÷�Ӧ��������Һ���������������Ƕ��٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(6��)ij����С���һʪ��ұͭ���ŷŵķ�ˮ�к������������ͭ������Ⱦ�Ϊ�ⶨ�÷�ˮ�и���Ⱦ��ĺ�������ұͭ���ṩ������ˮ�IJο�������С���ͬѧ����������ʵ�顣ȡ��ˮ500g�������м���������������Ϊ20��������������Һ����ó��������������������������Һ��������ϵ����ͼ��
�����������ݼ��㣺
(1)500g�÷�ˮ������ͭ��������
(2)�÷�ˮ���������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ɽ��ʡ��̨�г���ѧҵˮƽ���Ի�ѧ�Ծ� ���ͣ�������
(6��)ij����С���һʪ��ұͭ���ŷŵķ�ˮ�к������������ͭ������Ⱦ�Ϊ�ⶨ�÷�ˮ�и���Ⱦ��ĺ�������ұͭ���ṩ������ˮ�IJο�������С���ͬѧ����������ʵ�顣ȡ��ˮ500g�������м���������������Ϊ20��������������Һ����ó��������������������������Һ��������ϵ����ͼ��
�����������ݼ��㣺
(1)500g�÷�ˮ������ͭ��������
(2)�÷�ˮ���������������������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com