

| Дкј¶ | ёЯЦРҝОіМ | Дкј¶ | іхЦРҝОіМ |
| ёЯТ» | ёЯТ»Гв·СҝОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СҝОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СҝОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СҝОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СҝОіМНЖјцЈЎ | іхИэ | іхИэГв·СҝОіМНЖјцЈЎ |
ҝЖДҝЈәіхЦР»ҜС§ АҙФҙЈә МвРНЈә
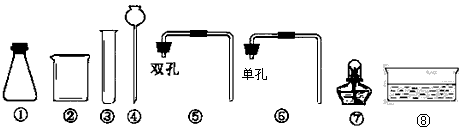
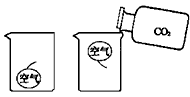
 КөСйКТіЈУГПВБРЧ°ЦГЦЖұё¶юСх»ҜМј
КөСйКТіЈУГПВБРЧ°ЦГЦЖұё¶юСх»ҜМјІйҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәіхЦР»ҜС§ АҙФҙЈә МвРНЈәҪвҙрМв
 КөСйКТіЈУГПВБРЧ°ЦГЦЖұё¶юСх»ҜМј
КөСйКТіЈУГПВБРЧ°ЦГЦЖұё¶юСх»ҜМјІйҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәіхЦР»ҜС§ АҙФҙЈәІ»Пк МвРНЈәМоҝХМв
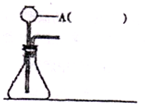
Ійҝҙҙр°ёәНҪвОц>>
№ъјКѧУУЕСЎ - Б·П°ІбБРұн - КФМвБРұн
әюұұКЎ»ҘБӘНшОҘ·ЁәНІ»БјРЕПўҫЩұЁЖҪМЁ | НшЙПУРәҰРЕПўҫЩұЁЧЁЗш | өзРЕХ©ЖӯҫЩұЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРәҰРЕПўҫЩұЁЧЁЗш | ЙжЖуЗЦИЁҫЩұЁЧЁЗш
ОҘ·ЁәНІ»БјРЕПўҫЩұЁөз»°Јә027-86699610 ҫЩұЁУКПдЈә58377363@163.com