
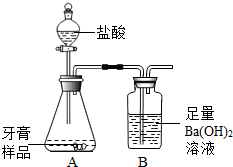
 ��ѧ��ȤС���ijƷ��������̼��ƺ�����������̽����������Ħ������Ҫ��̼��ơ�����������ɣ������ɷ���������ʱ���������ɡ���������ͼ��ʾװ�ã�ͼ�мг�������ȥ������ʵ�飬��ַ�Ӧ�ⶨB�����ɵ�BaCO3������������ȷ��̼��Ƶ�������������ش��������⡣
��ѧ��ȤС���ijƷ��������̼��ƺ�����������̽����������Ħ������Ҫ��̼��ơ�����������ɣ������ɷ���������ʱ���������ɡ���������ͼ��ʾװ�ã�ͼ�мг�������ȥ������ʵ�飬��ַ�Ӧ�ⶨB�����ɵ�BaCO3������������ȷ��̼��Ƶ�������������ش��������⡣
��1��װ��A��������Ʒ������ �� �����������ƣ��У����������װ�� �� �����������ƣ��У�A�����ٷ��� �� �������֣�����Ӧ��д������һ����Ӧ�Ļ�ѧ����ʽ �� ��
��2�����ⶨֵ��ʵ��ֵС�����ܵ�ԭ���� �� ��������ĸ��
a��װ��A��ˮ������HCl�Ƚ���װ��B�� b��װ��A��CO2δ��ȫ����װ��B
c��CO2�����ٶ�̫�쵼��Ba(OH)2δ��ȫ���� d������δ�μӹ���
��3��Ϊ�������ֲ�ȡ����һЩ��ʩ��
����A��B֮������һ��װ�� �� ���ѧʽ����Һ��ϴ��ƿ������������ �� _��
�ڽ�װ��B�еij������·�����һ���������ݣ�������� �� ��
����װ��B�ĺ���������һ��װ�� �� ����ҩƷ���ƻ�ѧʽ������ĸ���ܣ�Ŀ���� �� ��
��4��ȷ��ȡ���ݸ�16.00 g����Ʒ�����вⶨ�����BaCO3�����ֱ�Ϊ3.96 g��3.92 g��3.94 g���������Ʒ��̼��Ƶ�����������
�� ����д��������̣�4�֣�
��5��ijͬѧ�������������ⶨ�����и�Ԫ�ص�����������С��ͬѧ��Ϊ��ʹ�ų�ʵ�������Ͳ�����Ӱ�죬�����ⶨ�Ľ�����п���ƫС�������� �� ��
��1����ƿ ��Һ©�� 2 CaCO3+2HCl=CaCl2+H2O+CO2����Al(OH)3+3HCl=AlCl3+3H2O
��2���ڢۢ�
��3����AgNO3����ȥHCl���� �ڶ�����̼����Ч�� �ۼ�ʯ�һ������Ƶ� ��ֹ�����е�CO2����װ��B
��4�� ���̼�ᱵ������Ϊ��3.96g +3.92g +3.94g����3= 3.94g ��1�֣�
�μӷ�Ӧ��̼�������Ϊ2g��2�֣�
̼��Ƶ���������Ϊ12.5%��1�֣�
��5�������п��ܺ��иƵ���������������������𰸣���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ѧ��ȤС���ijƷ��������̼��ƺ�����������̽����
��ѧ��ȤС���ijƷ��������̼��ƺ�����������̽�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| Ʒ����XX��ů������ �ɷ֣��������ʣ����ۡ�ˮ������̿��ʳ�Ρ���ʯ�� �ڴ����ϣ������IJ� ������ϣ��������� �÷�����ʱ��ǰ�������ȡ���ڴ���ֱ�������·��ϣ����������ů12Сʱ�� |
 ��ʵ��һ����װ�����۲�������ʣ�����������ҪΪ��ɫ��ĩ�����л��С�����ɫ������С����
��ʵ��һ����װ�����۲�������ʣ�����������ҪΪ��ɫ��ĩ�����л��С�����ɫ������С����| ���� | ���� | ���� |
| ȡ�������������ĺ�ɫ��ĩ��ˮ�ܽ����ˣ�ȡ������Һ�����Թ��У��μ����� ������ ������ ��Һ��ϡ���� ϡ���� �����������ƣ� |
�� ������ɫ���� ������ɫ���� ���� ��ɫ�������ܽ� ��ɫ�������ܽ� �� |
�����Ȼ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ��� | ��һ�� | �ڶ��� | ������ | ������ |
| ����������g�� | 7.86 | 7.88 | 7.90 | 9.86 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
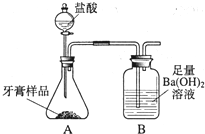
 ��2013?�����ж�ģ����ѧ��ȤС���ijƷ��������̼��ƺ�����������̽����������Ħ������Ҫ��̼��ơ�����������ɣ������ɷ���������ʱ���������ɣ���������ͼ��ʾװ�ã�ͼ�мг�������ȥ������ʵ�飬��ַ�Ӧ�ⶨB�����ɵ�BaCO3������������ȷ��̼��Ƶ�������������ش��������⣮
��2013?�����ж�ģ����ѧ��ȤС���ijƷ��������̼��ƺ�����������̽����������Ħ������Ҫ��̼��ơ�����������ɣ������ɷ���������ʱ���������ɣ���������ͼ��ʾװ�ã�ͼ�мг�������ȥ������ʵ�飬��ַ�Ӧ�ⶨB�����ɵ�BaCO3������������ȷ��̼��Ƶ�������������ش��������⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ѧ��ȤС���ijƷ��������̼��ƺ�����������̽����������Ħ������Ҫ��̼��ơ�����������ɣ������ɷ���������ʱ���������ɣ���������ͼ��ʾװ�ã�ͼ�мг�������ȥ������ʵ�飬��ַ�Ӧ�ⶨB�����ɵ�BaCO3������������ȷ��̼��Ƶ�������������ش��������⣮
��ѧ��ȤС���ijƷ��������̼��ƺ�����������̽����������Ħ������Ҫ��̼��ơ�����������ɣ������ɷ���������ʱ���������ɣ���������ͼ��ʾװ�ã�ͼ�мг�������ȥ������ʵ�飬��ַ�Ӧ�ⶨB�����ɵ�BaCO3������������ȷ��̼��Ƶ�������������ش��������⣮�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com