
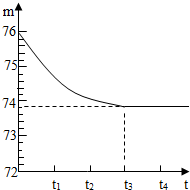
��6�֣���ʡ���ض��зḻ��ʯ��ʯ�����Դ����һ��ɽ�ϵ�ʯ��ʯ��Ʒ������ֻ�������������ʣ�����������һ�ּȲ�����ˮҲ�������ᷴӦ�����µĹ��壩��С�պ�����ͬѧ��ⶨ����Ʒ��̼��Ƶĵ���������������ȡ��һ��ʯ��ʯ��Ʒ�������ô����Ƴ�6g�����ձ��ڣ��ձ�������Ϊ20g����Ȼ�����50gijһ����������������������ϡ���ᣬ�ò��������������ڲ�������Ϊֹ����Ӧ����ʱ�䣨t�����ձ�������ʢ���ʵ���������m���Ĺ�ϵ����ͼ��ʾ��

�Իش�
��1����ʯ��ʯ��Ʒ�ô�������ҪĿ���� ��
��2��ʵ��������ų����ٶ�����̼��
��3����ʯ��ʯ��Ʒ��̼��Ƶ����������Ƕ��٣�������������1λС����
��1������Ӵ�������ӿ췴Ӧ����
��2��2.2g ��3��83.3%
��������
�����������1��Ϊ�˱�����Ʒ��̼��Ʋ�����ɷ�Ӧ���ɰ���Ʒ���飬������������Ʒ������ĽӴ������ʹ��Ӧ����֣�ͬʱ�����Լӿ췴Ӧ�����ʡ�
��2����ͼ���Կ�������t3ʱ��ʯ��ʯ��Ʒ�е�̼�������ȫ��Ӧ����ʣ������������Ϊ73.8g�����������غ㶨�ɣ���֪�ų����������̼������=20g+6g+50g��73.8g=2.2g��
(3) �������ɵĶ�����̼����������ϻ�ѧ����ʽ�ж�����̼��̼��Ƶ������ȣ��ɼ������ȡ��Ʒ��̼��Ƶ������������̼�������������Ʒ�����������������Ʒ��̼�������������
��6gʯ��ʯ��Ʒ������̼��Ƶ�����ΪX����
CaCO3+2HCl = CaCl2 + CO2��+H2O
100 44
x 2.2g
100��44=x��2.2g
���x="5g"
���Ը�ʯ��ʯ��Ʒ��̼��Ƶ���������=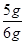 ��100%="83.3%"
��100%="83.3%"
�𣺸�ʯ��ʯ��Ʒ��̼��Ƶ�����������83.3%��
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���
�����������ǹ��ڻ�ѧ����ʽ�ļ����⣬��Ҫ������ͼ������Ӧ����ʽ�������ͽ����ѧ�����е��й����⣬Ҫ��ѧ��Ҫ�н�ǿ�����ݷ�������������Ĺؼ���Ҫ�����������غ㶨�ɣ��ɷ�Ӧǰ�������ı仯�������Ӧ�ų��Ķ�����̼��������Ȼ���ٸ�����صĻ�ѧ��Ӧ����ʽ����������֪����δ֪��Ӧ�������������㼴�ɡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
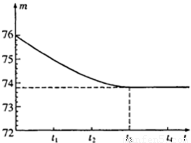
 ��2005?ɽ������ʡ���ض��зḻ��ʯ��ʯ�����Դ����һ��ɽ�ϵ�ʯ��ʯ��Ʒ������ֻ�����ʶ������裨����������һ�ּȲ�����ˮҲ�������ᷴӦ�����µĹ��壩��С�պ�����ͬѧ��ⶨ����Ʒ��̼��Ƶ���������������ȡһ��ʯ��ʯ��Ʒ���������Ƴ�6g�����ձ��ڣ��ձ�������Ϊ20g����Ȼ�����50g����ϡ���ᣬ�ò��������������ٲ�������Ϊֹ����Ӧ����ʱ�䣨t�����ձ�������ʢ���ʵ���������m���Ĺ�ϵ��ͼ��ʾ��
��2005?ɽ������ʡ���ض��зḻ��ʯ��ʯ�����Դ����һ��ɽ�ϵ�ʯ��ʯ��Ʒ������ֻ�����ʶ������裨����������һ�ּȲ�����ˮҲ�������ᷴӦ�����µĹ��壩��С�պ�����ͬѧ��ⶨ����Ʒ��̼��Ƶ���������������ȡһ��ʯ��ʯ��Ʒ���������Ƴ�6g�����ձ��ڣ��ձ�������Ϊ20g����Ȼ�����50g����ϡ���ᣬ�ò��������������ٲ�������Ϊֹ����Ӧ����ʱ�䣨t�����ձ�������ʢ���ʵ���������m���Ĺ�ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʡ���ض��зḻ��ʯ��ʯ�����Դ����һ��ɽ�ϵ�ʯ��ʯ��Ʒ������ֻ��������������(����������һ�ּȲ�����ˮҲ�������ᷴӦ�����µĹ���)��ʵ��ѧУ��ͬѧ����ⶨ����Ʒ��̼��Ƶ��������������Dz�ȡ��һ��ʯ��ʯ��Ʒ����������Ƴ�6g�����ձ���(�ձ�����Ϊ20g)��Ȼ�����50gijһ����������������ϡ���ᣬ�ò��������������ٲ�������Ϊֹ����Ӧ����ʱ�䣨t�����ձ�������ʢ������������m���Ĺ�ϵ����ͼ��ʾ���Իش�

(1)��ʯ��ʯ��Ʒ�ô�������ҪĿ����__________________________________________��
(2)ʵ�����ʱ�����ų����ٶ�����̼?
(3)��ʯ��ʯ��Ʒ��̼��Ƶ����������Ƕ���?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ�ൺ�н�����������ѧ�п���ѧģ���Ծ��������������棩 ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2005��ɽ��ʡ�п���ѧ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�꽭��ʡ�������������и����п���ѧģ���Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com