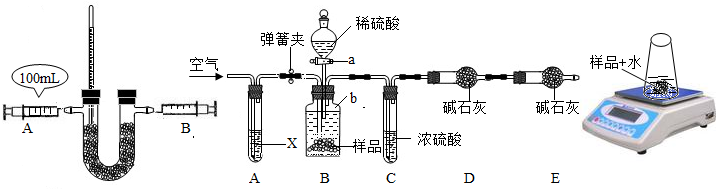
�⣺��1������������£���DZͧ�﹤������Ա���õ���ɫ�Ĺ������ƹ����ڳ����¼����������̼��Ӧ���������������ַ������������ŵ��ǣ���Ӧ�����͡�����Я������������Ⱦ����������ͬʱ�������˶�����̼��
��2���ټ��װ�������Եķ������ƶ�Aע�����Ļ�������B�л��������ƶ������ռ������������ͬ�������������ã�
����U�ι���ʢװ������Na
2O
2ҩƷ��ע������Ϊ������̼���壬���Ӻ�װ�ã�������������£���DZͧ�﹤������Ա�����������ҩƷ������ԭ��ȷ��������̼��ȫ��Ӧ���õ�������������
�����¶ȼ���ʾ�˷�Ӧ�ų�������˵���˷�ӦΪ���ȷ�Ӧ��ͬʱ�����Թ۲쵽����ɫ�Ĺ����ĩ��Ϊ��ɫ������
����Ϊ��ͬ�������£���ͬ��������������ȵķ�����������2Na
2O
2+2CO
2=2Na
2CO
3+O
2����������̼���������������2��1�����Aע�����ж�����̼���巴Ӧ��ȫ��װ�ûָ������£�Bע�����л���Ӧͣ���� 50mL����
��3��Na
2O
2¶���ڿ��������������ʣ���Ӧ�Ļ�ѧ����ʽΪ��2Na
2O
2+2CO
2=2Na
2CO
3+O
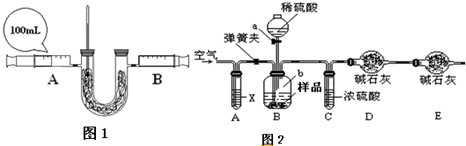
2����ʵ�鷽����������ʵĹ��̣�ȡ��Ʒ�������Թ��У�����ϡ���ᣬ���������壬ͨ��ʯ��ˮ��ʯ��ʯ����ǣ�˵���ѱ��ʣ�
iʢ��ϡ�����ʵ����������Ϊ��Һ©��
ii���ܢ�������ʵ�����̫�죬���ɵĶ�����̼���ᱻ��ʯ����ȫ���գ������̼����������ʵ�������٣���ᵼ�²ⶨ��Na
2O
2�Ĵ���ƫ��
iii���������Ŀ���ǽ�װ���в����Ķ�����̼�ij���ʹ��Ӧ��֣�Ϊ�����յ������ж�����̼��װ��A���Լ�XӦѡ�� NaOH��Һ��
ivEװ�õ������Ƿ�ֹ�����еĶ�����̼��ˮ��������Dװ�ã�Ӱ��ʵ������
��4��i����ϸ�۲��ϱ����ݼ����ж�20gNa
2O
2��20mLˮ��ȫ��Ӧ��ʱ����240�룻
ii�����ݱ������ݼ�������ɵ�������������85g-81.8g=3.2g���ٸ���Na
2O
2��ˮ��Ӧ�Ļ�ѧ����ʽ��2Na
2O
2+2H
2O=4NaOH+O
2�����������Ʒ��Na
2O
2�Ĵ���Ϊ��78%��
�ʴ�Ϊ����1����Ӧ�����͡�����Я������������Ⱦ����������ͬʱ�������˶�����̼��д��һ�㼴�ɣ�
��2������һֻע�����г�ȡһ���������������װ�ã������ƶ�������һֻ���ܷ��ռ�������������壬����˵�����������ã�
��������̼��ȫת��Ϊ����������ʵ�������ȣ�����ɫ�ķ�ĩ��Ϊ��ɫ��ĩ�� 50mL��
��3��ȡ����������Ʒ�μ�ϡ���ᣬ������ͨ�����ʯ��ˮ�����ֻ���˵���ѱ��ʣ�
��Һ©����ƫ��װ���в����Ķ�����̼�ij���ʹ��Ӧ��֣�NaOH��Һ����ֹ�����еĶ�����̼��ˮ��������Dװ�ã�
��4��240
���̣�
������� ����������Ϊ 20g+45g+20g-81.80g=3.2g
�裺Na
2O
2������Ϊx
2Na
2O
2+2H
2O�T4NaOH+O
2��
156 32
x 3.2g

��� x=15.6g
����Na
2O
2�Ĵ���=

=78%
��������1���ӷ�Ӧ��������Ӧ���������ȷ��������Ӧ���ŵ㣻
��2���ٸ���װ���ص�̽��װ�������Եļ�鷽��������U�ι���ʢװ������Na
2O
2ҩƷ��ע������Ϊ������̼���壬���Ӻ�װ�ã�������������£���DZͧ�﹤������Ա�������̽��ҩƷΪʲô������������¶ȼ���ʾ�˷�Ӧ�ų�������ͬʱ�����Թ۲쵽����ɫ�Ĺ����ĩ��Ϊ��ɫ��
��3������Na
2O
2¶���ڿ��������������̼��Ӧ����̼���Ʒ������ʣ����ʵ�飬��֤Na
2O
2�Ƿ���ʣ�����װ�õ��ص�ͷ�Ӧԭ������ʵ����̣�
��4��a����ϸ�۲��ϱ����ݼ����ж�20gNa
2O
2��20mLˮ��Ӧ��ʱ�䣻b�����ݱ������ݼ�������ɵ��������������ٸ���Na
2O
2��ˮ��Ӧ�Ļ�ѧ����ʽ�������Ʒ��Na
2O
2�Ĵ��ȣ�
����������̽���������ƹ����ڳ������������̼��Ӧԭ����������;��֪ʶ�㣬�漰֪ʶ��ܹ㣬̽������϶࣬�Ƕ���ѧ��������һ�����ɶ�õĺ��⣮

 ��� x=15.6g
��� x=15.6g =78%
=78%