



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ���� | ���㡢���ȡ����ǡ����⡢���㡢�뵰�� |
| ���� | �С�ʳ�Ρ����ǡ����˾Ƶ� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)��������Ȫ�ȼٴ����������еĺ�ȥ����
����Ȫˮ�����ء��ơ�þ��������ȣ�����ġ��ء��ơ�þ�������衱ָ����________(����ĸ)��
A������ B��ԭ�� C��Ԫ��
����Ȫˮ��pH��7.5��8.9֮�䣬����Ȫˮ��______(����ԡ��������ԡ������ԡ�)��
�ۼ������Ȫˮ��Ӳˮ������ˮ�ļ�����
________________________________________________________________________��
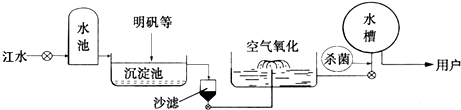
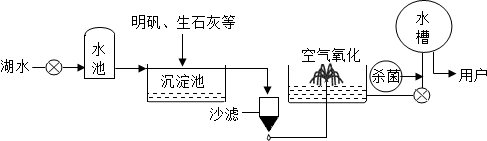
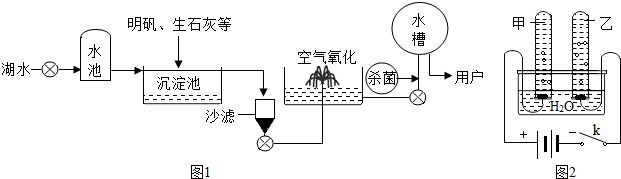
(2)ƽ̶����컡���ɫ���˵ġ���ʮ���ź�����ƽ̶����ˮ����ˮԴ������ˮ��������������ͼ��ʾ��

��������������ˮ����ʹ�õľ�ˮ������________(����ĸ)��
A������ B������
C����� D������
���ڳ������У�������ʯ�ҿɽ���ˮ��Ӳ�ȡ���ʯ����ˮ��Ӧ�Ļ�ѧ����ʽΪ��________________________________________________________________________��
������ˮ����������������ɱ���������뽫������ˮ��Ӧ�Ļ�ѧ����ʽ����������
Cl2��H2O===HClO��________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com