AЎўBЎўCЎўDЎўEКЗіхЦР»ҜС§ЦРөДОеЦЦіЈјыОпЦКЈ®CЎўDКЗөҘЦКЈ¬CОӘЧПәмЙ«№ММеЈ¬DКЗГЬ¶ИЧоРЎөДЖшМеЈ¬BөДЕЁИЬТәіЈУГЧчёЙФпјБЈ®ТСЦӘFeәНAЎўBөДЛ®ИЬТә·ЦұрДЬ·ўЙъ·ҙУҰЈә
ўЩFe+AЎъC+EЈ»ўЪFe+BЎъD+E
ЈЁ1Ј©РҙіцAЎўBЎўCөД»ҜС§КҪЈәA
CuSO4
CuSO4
B
H2SO4
H2SO4
C
Cu
Cu
ЈЁ2Ј©ПЦУРFeәНAЎўCИэЦЦ№ММеЧйіЙөД»мәПОпЈ¬Ді»ҜС§РЛИӨРЎЧйөДН¬С§ПлНЁ№эКөСйІв¶ЁёГ»мәПОпЦРCОпЦКөДЦКБҝ·ЦКэЈ®
ЎҫЙијЖКөСйЎҝИзНјЛщКҫ
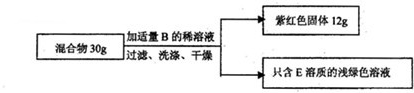
ЎҫКэҫЭҙҰАнЎҝ
ўЩРЎёХёщҫЭКэҫЭјЖЛгіц»мәПОпЦКЦРCОпЦКөДЦКБҝ·ЦКэОӘЈә
ЎБ100%Ј¬РЎЗҝИПОӘКэҫЭҙҰАнІ»ХэИ·Ј¬ФӯТтКЗ
12gЧПәмЙ«№ММеЦР°ьә¬УРМъәНБтЛбНӯ·ҙУҰЙъіЙөДНӯЈЁМъәНБтЛбНӯ·ҙУҰЙъіЙБЛНӯ»т12gЧПәмЙ«№ММеЈ¬І»Ц»КЗФӯАҙөДCОпЦКЈ¬»т12gЧПәмЙ«№ММеөДЦКБҝҙуУЪФӯАҙCОпЦКөДЦКБҝЈ©Ј®
12gЧПәмЙ«№ММеЦР°ьә¬УРМъәНБтЛбНӯ·ҙУҰЙъіЙөДНӯЈЁМъәНБтЛбНӯ·ҙУҰЙъіЙБЛНӯ»т12gЧПәмЙ«№ММеЈ¬І»Ц»КЗФӯАҙөДCОпЦКЈ¬»т12gЧПәмЙ«№ММеөДЦКБҝҙуУЪФӯАҙCОпЦКөДЦКБҝЈ©Ј®
ўЪЛыГЗҫӯ№эИПХж·ЦОцәуЈ¬·ўПЦ»№ИұЙЩұШТӘөДКөСйКэҫЭЈ¬ҫӯ№эЦШРВКФСйЈ¬ІўУГМмЖҪіЖіц·ҙУҰЗ°әуЧЬЦКБҝјхЙЩБЛ0Ј¬1gЈ¬ҙУ¶шЛгіц»мәПОпЦРCОпЦКөДЦКБҝ·ЦКэОӘ
18.7%
18.7%
Ј®
ЎҫКөСй·ҙЛјЎҝ
Н¬С§ГЗ¶ФёГ»мәПОпөДіЙ·Цҫӯ№эИПХж·ЦОцәу·ўПЦЈ¬Ц»ёщҫЭОпЦКөДОпАнРФЦКТІДЬІв¶ЁCОпЦКөДЦКБҝ·ЦКэЈ¬КФРҙіцјтГчөДКФСй·Ҫ·Ё
Ҫ«ОпЦКИЬУЪЛ®Ј¬№эВЛЈ¬ПҙөУәжёЙЈ¬іЖөГВЛФьЦКБҝЈ®УГҙЕМъОьКХВЛФьЈ¬іЖөГКЈУаОпЦКөДЦКБҝјҙНӯөДЦКБҝЈ¬УГНӯөДЦКБҝіэТФЧЬЦКБҝјҙөГНӯөДЦКБҝ·ЦКэЈ®
Ҫ«ОпЦКИЬУЪЛ®Ј¬№эВЛЈ¬ПҙөУәжёЙЈ¬іЖөГВЛФьЦКБҝЈ®УГҙЕМъОьКХВЛФьЈ¬іЖөГКЈУаОпЦКөДЦКБҝјҙНӯөДЦКБҝЈ¬УГНӯөДЦКБҝіэТФЧЬЦКБҝјҙөГНӯөДЦКБҝ·ЦКэЈ®
Ј®




 ЦұНЁ№уЦЭГыРЈЦЬІвФВҝјЦұНЁГыРЈПөБРҙр°ё
ЦұНЁ№уЦЭГыРЈЦЬІвФВҝјЦұНЁГыРЈПөБРҙр°ё
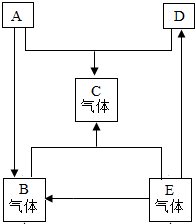 ТСЦӘAЎўBЎўCЎўDЎўEКЗіхЦР»ҜС§іЈјыөДОеЦЦОпЦКЈ¬ЖдЦРAЎўDКЗәЪЙ«№ММеЈ¬AОӘәЪЙ«№ММеөҘЦКЈ¬DОӘәЪЙ«№ММеСх»ҜОпЈ¬ЗТЖдЦРә¬УРИЛАаК№УГЧо№г·әөДҪрКфФӘЛШЈ¬BЎўCЎўEКЗОЮЙ«ЖшМеЈ¬Ј®ЛьГЗФЪТ»¶ЁМхјюПВөДЧӘ»Ҝ№ШПөИзПВНјЛщКҫЈЁЖдЛы·ҙУҰОпәНЙъіЙОпТСВФИҘЈ©Ј®
ТСЦӘAЎўBЎўCЎўDЎўEКЗіхЦР»ҜС§іЈјыөДОеЦЦОпЦКЈ¬ЖдЦРAЎўDКЗәЪЙ«№ММеЈ¬AОӘәЪЙ«№ММеөҘЦКЈ¬DОӘәЪЙ«№ММеСх»ҜОпЈ¬ЗТЖдЦРә¬УРИЛАаК№УГЧо№г·әөДҪрКфФӘЛШЈ¬BЎўCЎўEКЗОЮЙ«ЖшМеЈ¬Ј®ЛьГЗФЪТ»¶ЁМхјюПВөДЧӘ»Ҝ№ШПөИзПВНјЛщКҫЈЁЖдЛы·ҙУҰОпәНЙъіЙОпТСВФИҘЈ©Ј® 23ЎўТСЦӘAЎўBЎўCЎўDЎўEКЗіхЦР»ҜС§АпіЈјыөДОеЦЦОпЦКЈ¬ЛьГЗФЪТ»¶ЁМхјюПВДЬ·ўЙъИзНјЛщКҫөДЧӘ»ҜЈ¬ЖдЦР·ҙУҰўЩКЗёҙ·ЦҪв·ҙУҰЈ¬EКЗФміЙОВКТР§УҰөДЦчТӘЖшМеЈ®
23ЎўТСЦӘAЎўBЎўCЎўDЎўEКЗіхЦР»ҜС§АпіЈјыөДОеЦЦОпЦКЈ¬ЛьГЗФЪТ»¶ЁМхјюПВДЬ·ўЙъИзНјЛщКҫөДЧӘ»ҜЈ¬ЖдЦР·ҙУҰўЩКЗёҙ·ЦҪв·ҙУҰЈ¬EКЗФміЙОВКТР§УҰөДЦчТӘЖшМеЈ® AЎўBЎўCЎўDЎўEКЗіЈјыОпЦКЈ¬ЖдЦРBәНEКЗҝХЖшЦРБҪЦЦЖшМеЈ¬CКЗМъРвЦчТӘіЙ·ЦЈ®ИзНјЛщКҫЈ¬ЦұПЯұнКҫПа»ҘјдДЬ№»·ҙУҰЈ¬јэН·ұнКҫЧӘ»ҜөД·ҪПтЈ®
AЎўBЎўCЎўDЎўEКЗіЈјыОпЦКЈ¬ЖдЦРBәНEКЗҝХЖшЦРБҪЦЦЖшМеЈ¬CКЗМъРвЦчТӘіЙ·ЦЈ®ИзНјЛщКҫЈ¬ЦұПЯұнКҫПа»ҘјдДЬ№»·ҙУҰЈ¬јэН·ұнКҫЧӘ»ҜөД·ҪПтЈ® ЈЁ2013?РмЦЭ¶юДЈЈ©AЎўBЎўCЎўDЎўEКЗіхЦР»ҜС§ЦРіЈјыөДІ»Н¬АаұрөДОпЦКЈЁОпЦК°ҙөҘЦКЎўСх»ҜОпЎўЛбЎўјоЎўСО·ЦАаЈ©Ј®ТСЦӘAКЗөҘЦКЈ»CКЗәмЧШЙ«№ММеЈ»EКЗЛ®ИЬТәҝЙК№·УМӘКФТәұдОӘәмЙ«өДСОЈ®НјЦРЎ°-ЎұұнКҫПаБ¬өДОпЦКБҪБҪЦ®јдҝЙТФ·ўЙъ·ҙУҰЈ¬Ў°ЎъЎұұнКҫУЙДіТ»ОпЦКҝЙЦЖөГБнТ»ОпЦКЈЁІҝ·Ц·ҙУҰОпЎўЙъіЙОпј°·ҙУҰМхјюТСВФИҘЈ©Ј®»ШҙрПВБРОКМвЈә
ЈЁ2013?РмЦЭ¶юДЈЈ©AЎўBЎўCЎўDЎўEКЗіхЦР»ҜС§ЦРіЈјыөДІ»Н¬АаұрөДОпЦКЈЁОпЦК°ҙөҘЦКЎўСх»ҜОпЎўЛбЎўјоЎўСО·ЦАаЈ©Ј®ТСЦӘAКЗөҘЦКЈ»CКЗәмЧШЙ«№ММеЈ»EКЗЛ®ИЬТәҝЙК№·УМӘКФТәұдОӘәмЙ«өДСОЈ®НјЦРЎ°-ЎұұнКҫПаБ¬өДОпЦКБҪБҪЦ®јдҝЙТФ·ўЙъ·ҙУҰЈ¬Ў°ЎъЎұұнКҫУЙДіТ»ОпЦКҝЙЦЖөГБнТ»ОпЦКЈЁІҝ·Ц·ҙУҰОпЎўЙъіЙОпј°·ҙУҰМхјюТСВФИҘЈ©Ј®»ШҙрПВБРОКМвЈә AЎўBЎўCЎўDЎўEКЗіхЦР»ҜС§ЦРіЈјыөД5ЦЦОпЦКЈ¬ЛьГЗ¶јә¬УРТ»ЦЦПаН¬өДФӘЛШЈ¬ИзНјұнКҫёчОпЦКЦ®јдөДЧӘ»Ҝ№ШПөЈ®ЖдЦРЈ¬AОӘКіСОөДЦчТӘіЙ·ЦЈ¬BЦРә¬ұөФӘЛШЈ¬DЎўEөДИЬТә¶јУРСХЙ«Ј¬
AЎўBЎўCЎўDЎўEКЗіхЦР»ҜС§ЦРіЈјыөД5ЦЦОпЦКЈ¬ЛьГЗ¶јә¬УРТ»ЦЦПаН¬өДФӘЛШЈ¬ИзНјұнКҫёчОпЦКЦ®јдөДЧӘ»Ҝ№ШПөЈ®ЖдЦРЈ¬AОӘКіСОөДЦчТӘіЙ·ЦЈ¬BЦРә¬ұөФӘЛШЈ¬DЎўEөДИЬТә¶јУРСХЙ«Ј¬