
 ȫ�ų��100��ϵ�д�
ȫ�ų��100��ϵ�д� Ӣ�ŵ��ϵ�д�
Ӣ�ŵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| \ | A | B | C | D |
| ����� | ���������� | �ǽ������� �ǽ������� |
�� | �� �� |
| �����ڸ��������� | CO2 CO2 |
Cu | Fe��OH��3 Fe��OH��3 |
H2O |
| ���� |
| Ҷ���� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������2012���п��������⻯ѧ���� ���ͣ�058
ѧϰ�����ѧϰ�����dzɲŵıر�������������ղ�ͬ��ѧϰ��������ȡ���°빦����Ч�����������·�������������⣺
(1)���෨����������4������
A��ZnO��MgO��CO2��Na2O
B��Cu��N2��O2��Cl2
C��KNO3��NaHCO3��KClO3��Fe(OH) 3
D��H2SO4��H2O��HCl��HNO3
�밴Ҫ����д�±��հ�(��д���ʵĻ�ѧʽ������)
(2)�Աȷ���
A��ͨ����CO2��H2O![]() H2CO3��6CO2��6H2O
H2CO3��6CO2��6H2O![]() C6H12O6��6O2������ѧ��Ӧ�ĶԱȣ����ܵó��Ľ�����________��
C6H12O6��6O2������ѧ��Ӧ�ĶԱȣ����ܵó��Ľ�����________��
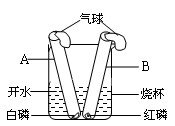
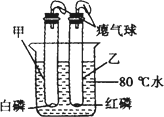
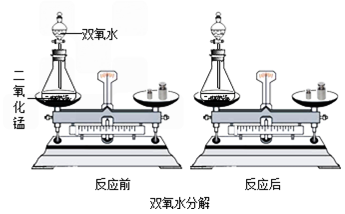
B������ͼ��ʾ��С��ͬѧ��ʢ�и�����ļ��Թܺ���������Թܲ���ʢ80 tˮ���ձ���(�ס����Թܾ����������ܷ�)����һ����ּ��Թ��еİ���ȼ�գ����Թ��еĺ���û��ȼ�գ������С��ͬѧ��ʵ�����Աȵó���ȼ��ȼ�����������֮һ��________��
(3)���������
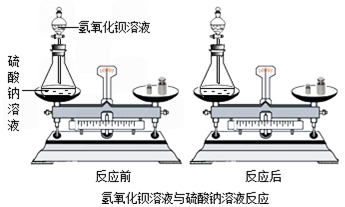
С��ͬѧͨ���Դ�����ѧ��Ӧ������������Һ����������Һ��˫��ˮ�ֽ�(����ͼ)��Ӧǰ������ʵ������ܺ͵IJⶨ���ó��μӻ�ѧ��Ӧ�ĸ����ʵ������ܺ�________(����ڡ�����С�ڡ�����)��Ӧ�����ɵĸ����ʵ������ܺͣ�
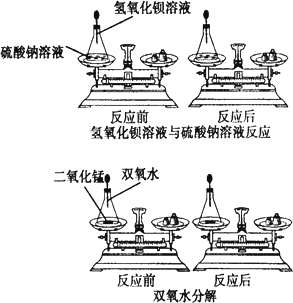
����С���ó��Ľ��ۣ����Ƴ�7.9 g������ؼ���һ���ʣ���������Ϊ7.5 g����Ӧ��������������Ϊ________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���п����� ���ͣ�ʵ����

 C6H12O6+6O2
C6H12O6+6O2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ�������п���ѧ�Ծ��������棩 ���ͣ������
| \ | A | B | C | D |
| ����� | ���������� | �� | ||
| �����ڸ��������� | Cu | H2O |
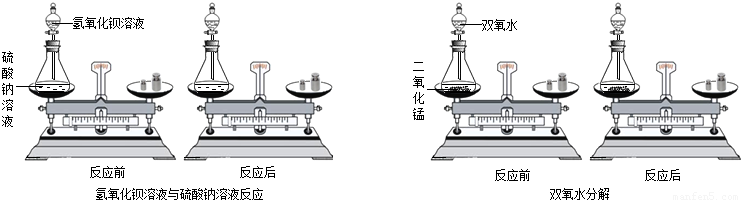
 C6H12O6+6O2������ѧ��Ӧ�ĶԱȣ����ܵó��Ľ����� ��
C6H12O6+6O2������ѧ��Ӧ�ĶԱȣ����ܵó��Ľ����� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com