
 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
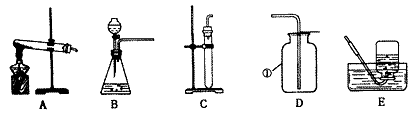
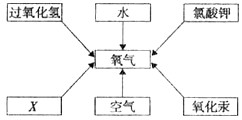
��ͼ�ǻ�ѧʵ������ȡ����ij���װ�á�
(1)д��ͼ�б�����������ƣ��� ���� ��
(2)�������������dz��н̲��г��ֵĻ�ѧ���ʣ���һ�������£������Բ���������
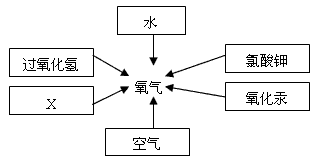
��д����ͼ������X������ ��ʵ�����ø�ҩƷ��ȡ�����Ļ�ѧ����ʽΪ ����ѡ�õķ���װ��Ϊ (�����)���ռ�װ��Ϊ (�����)�����������ļ�㷽���� ��
(3)ʵ������ȡ������̼�ķ���װ���� (�����)���䷴Ӧ��ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ȫ���п����������ר���� ������Ʊ���һ�� ���ͣ������
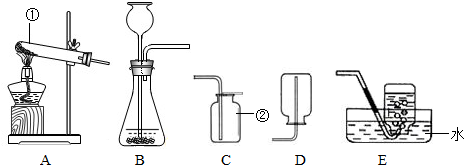
��10��������16������15�֣���ͼ�ǻ�ѧʵ������ȡ����ij���װ�á�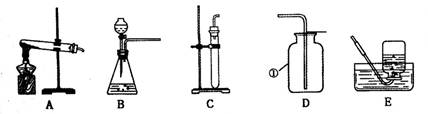
��1����д��ͼ�б��Тٵ��������ƣ� ��
��2���������������dz��н̲��г��ֵĻ�ѧ���ʣ���һ�������£������Բ���������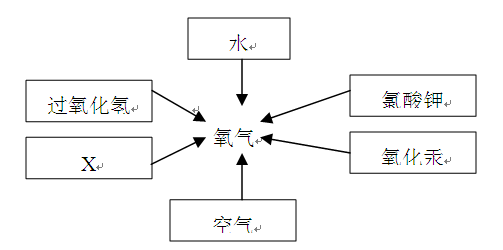
����д����ͼ������X������ ��ʵ�����ø�ҩƷ��ȡ�����Ļ�ѧ����ʽΪ�� ����Ҫ��ȡ�ϴ�������������ʵ��װ��Ӧѡ �� �����ţ���
��3��ʵ������B��Cװ�ÿ���ȡ ���壨��һ�ּ��ɣ�����ȡ������Ļ�ѧ����ʽΪ ��װ��B��C�Ƚϣ�B���ŵ��ǣ� ��дһ�㣩��
��4���������ƣ�Na2O2�������ڳ�������ˮ��ӦҲ���Բ���������ͬʱ�����������ơ�д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ȫ���п����������ר����������Ʊ���һ�� ���ͣ������
��10��������16������15�֣���ͼ�ǻ�ѧʵ������ȡ����ij���װ�á�

��1����д��ͼ�б��Тٵ��������ƣ� ��
��2���������������dz��н̲��г��ֵĻ�ѧ���ʣ���һ�������£������Բ���������
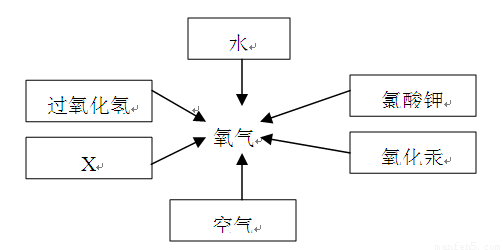
����д����ͼ������X������ ��ʵ�����ø�ҩƷ��ȡ�����Ļ�ѧ����ʽΪ�� ����Ҫ��ȡ�ϴ�������������ʵ��װ��Ӧѡ �� �����ţ���
��3��ʵ������B��Cװ�ÿ���ȡ ���壨��һ�ּ��ɣ�����ȡ������Ļ�ѧ����ʽΪ ��װ��B��C�Ƚϣ�B���ŵ��ǣ� ��дһ�㣩��
��4���������ƣ�Na2O2�������ڳ�������ˮ��ӦҲ���Բ���������ͬʱ�����������ơ�д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�п����� ���ͣ�ʵ����
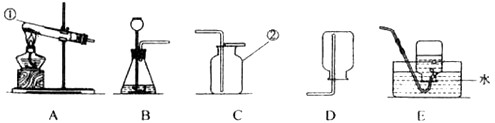
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com