�⣺��3�����ݽ��ۣ����ֱ��ʣ���ô�ɷ����������ƺ�̼��ƣ��ʡ��ڵ�ȥ�ϲ���Һ�������Թ���ע��ϡ���ᡱ��϶������ݲ�����
��û�����ݲ�����˵����������δ���ʣ���ȫ�����������ƣ��ʢ�ȡ�ϲ���Һ��������ɫ��̪��Һ����ɫ��̪��Һ��죻
��4���������Ʊ����������̼��Ӧ����̼��ƺ�ˮ��̼��ƺ����ᷴӦ����������̼���壬�ʷ�Ӧ�ķ���ʽΪ��Ca��OH��
2+CO
2=CaCO
3��+H
2O��CaCO
3+2HCl=CaCl
2+H
2O+CO
2����
��5�������ʯ��ˮ��ϡ���ᷴӦû���������������Ӧ�÷�̪����ɫ�仯ָʾ��Һ����Եı仯�Ӷ��жϷ�Ӧ�Ľ��м���Ӧ�ij̶ȣ��ʴ�Ϊ��
�ڳ����ʯ��ˮ�еμӼ��η�̪��Һ����Һ���ɫ��Ȼ����εμ�ϡ���ᣬ��ɫ����ʧ����֤�������ʯ��ˮ��ϡ���ᷴӦ��
����ɫǡ�ñ�Ϊ��ɫʱ������ǡ����ȫ��Ӧ��
��6���⣺���ɶ�����̼������Ϊ��20g+100g-117.8g=2.2g
��μӷ�Ӧ��̼��Ƶ�����ΪX��
CaCO
3+2HCl=CaCl
2+H
2O+CO
2��
100 44
X 2.2g
100/44=X/2.2g X=5 g
�������ƵĴ���=
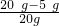
��100%=75%
���������ƵĴ�����75%��
�ʴ�Ϊ����3��ʵ��̽�����������ݲ��������ֱ��ʣ�����ɫ��̪��Һ��죻û�б��ʣ�
��4��С����˼����Ca��OH��
2+CO
2=CaCO
3��+H
2O��CaCO
3+2HCl=CaCl
2+H
2O+CO
2��
��5���ڳ����ʯ��ˮ�еμӼ��η�̪��Һ����Һ���ɫ��Ȼ����εμ�ϡ���ᣬ��ɫ����ʧ����֤�������ʯ��ˮ��ϡ���ᷴӦ��
����ɫǡ�ñ�Ϊ��ɫʱ������ǡ����ȫ��Ӧ��
��6���⣺���ɶ�����̼������Ϊ��20g+100g-117.8g=2.2g
��μӷ�Ӧ��̼��Ƶ�����ΪX��
CaCO
3+2HCl=CaCl
2+H
2O+CO
2��
100 44
X 2.2g
100/44=X/2.2g X=5 g
�������ƵĴ���=
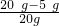
��100%=75%
���������ƵĴ�����75%��
��������3�������������Ƶı��ʳ̶ȷ�������û�б��ʣ�ֻ�����������ƣ��ӷ�̪��Һ�ῴ����̪��Һ��ɺ�ɫ����ϡ����û�����ݲ�����
�ڲ��ֱ��ʣ������������ƺ�̼��ƣ��ӷ�̪��Һ�ῴ����̪��Һ��ɺ�ɫ����ϡ���������ݲ�����
��ȫ�����ʣ�ֻ����̼��ƣ��ӷ�̪��Һ���ῴ����̪��Һ��ɺ�ɫ����ϡ���������ݲ�����
��4�������������Ʊ������������̼��Ӧ����̼��ƺ�ˮд����صķ���ʽ��
��5��ͨ����̪��Һ��ɫ�ı仯ָʾ��Ӧ�ķ�������Ӧ�ij̶ȣ�
��6�����ݷ�Ӧǰ����Һ����������������̼����������ͨ������ʽ����̼��Ƶ��������Ӷ�֪���������Ƶ����������������������Ĺ�ʽ�����������ƵĴ��ȣ�
���������⿼�����������Ƶı��ʣ����������Ʊ��ʵ�ԭ����������ơ�̼��Ƶ������ǽ������ǰ��ͻ��������⣬��Ҫ��������������ۣ����߸��ݽ����Ƴ�������ȷ��д��ѧ����ʽ��

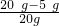 ��100%=75%
��100%=75% 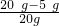 ��100%=75%
��100%=75% 

