��2012?��ţ��һģ��ע������һ����ͨ��ҽ����е�������ڻ�ѧʵ��װ���г��ֵ�Խ��Խ�࣬����ijЩʵ������Ĺ۲��ʵ����̵ĸĽ����������벻����Ч����
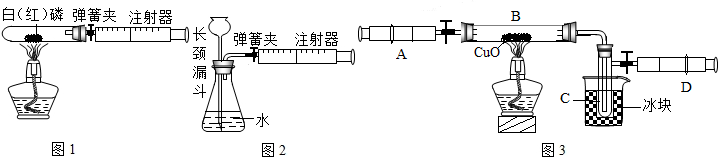
��1����ͼ1��50mL�Թ�����Ӧ��������ȼ�վ����ܱ���������У��ɷ�ֹ������Ⱦ��������50mLע�������������ȴ���15mL�̶ȴ���������ȼ�����ĵ����������
[����]��
�ټ��װ�õ������ԣ���װ��ҩƷ�������������ۼн����ɼУ����Ȱ��ף��۲��Թ���������������Ϊ
����ȼ�գ�������������
����ȼ�գ�������������
����ȼ�ս������Թ���ȴ����ɼУ����Կ��������������Ƶ�Լ
5
5
mL�̶ȴ���ȡ����ֵ����
��2��ͼ2������ע���������ķ������Լ��װ�õ������ԣ����ɼУ�������������������ʱ������ܹ۲쵽
D
D
��ѡ����ţ�����˵��װ�����������ã�
A��ע��������Һ�� B��ƿ��Һ������
C������©����Һ������ D������©���¶˹ܿڲ�������
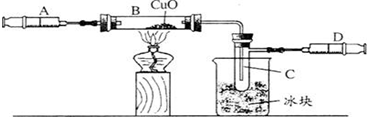
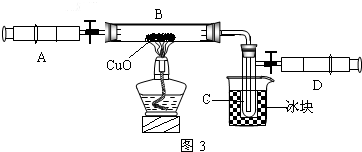
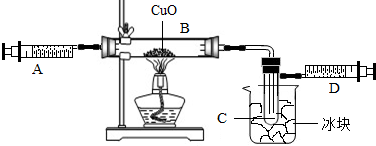
��3��ijѧ��Ϊ�˲ⶨ������Ԫ���γɵ���̬������X����ɣ�������ͼ3��ʾ��ʵ�飮����ע����A�е�����X��������װ��CuO��Bװ�ã�ʹ֮��ȫ��Ӧ���õ����½����
��B���к�ɫ��ĩ���
��ʵ��ǰB�ܼ�ҩƷ������Ϊ21.32g��ʵ���Ϊ21.16g��
��C�����ռ��������ʵ���ɵõ�H
2��O
2����D���ռ�������N
2��
��X����Ԫ�ص���������14��3���ʣ�
a�����ʵ��˵��������еĻ�ѧ������
��ԭ��
��ԭ��
��
b��C���ռ�����Һ�壬������
0.18
0.18
g��
c��B�з�Ӧ�Ļ�ѧ����ʽ��
��
�������ʵ���У���û�����ų��������еĿ������Ƿ����CO��ԭ����ͭ������ը��Ϊʲô��
���ᣬ��Ϊ��Ӧ���������û�п�ȼ���������
���ᣬ��Ϊ��Ӧ���������û�п�ȼ���������
��