�ڹ�������������������ʱ����������������Ҫ�����ã�
��1������������ɱ��ϸ���������ͼ������ѵȣ���������Ϊ______��
��2������������Һ�������˿�������������ø�Ĵ������£���������ɷ����ֽⷴӦ����ѧ����ʽΪ______��
��3���������ᣨCH3COOOH���DZ��㷺ʹ�õĸ�Ч�������������ȶ��ֽ�ų�һ�ֳ��������嵥�ʣ������ɴ��ᣨCH3COOH�����÷�Ӧ�Ļ�ѧ����ʽΪ______����һƿ19%�Ĺ���������Һ����ö���ȫ�ֽ⣬������Һ�д������������Ϊ______���������һλС������
��4��2008��5��12���봨�ش��������������ˮԴ������Ⱦ��Ϊ���ϵ���Ⱥ����ˮ��ȫ���й��������߲���ʹ���˶�������������ɱ������������Ҫ�У���ˮƬ��Na2FeO4�������Ⱦ���C3O3N3Cl2Na�����������ȣ�C1O2������84������Һ����Ч�ɷ�ΪNaClO����
����ȡNa2FeO4�ķ�ӦΪ��2Fe��OH��3+3NaClO+4NaOH=2Na2FeO4+3X+5H2O��X�Ļ�ѧʽΪ______��
�������й�˵����ȷ����______��
A������ɱ���Ĺ����ǻ�ѧ�仯��
B��C3O3N3Cl2Na����Է�������Ϊ232.5��
C��C3O3N3Cl2Na��C��OԪ�ص�������Ϊ3��4��
D��ClO2��NaClO��C3O3N3Cl2Na�о�����Ԫ�أ�ClO2����Ԫ�ص������������
����1�����������׳��ռ�������ƣ�
��2��2H
2O
2
2H
2O+O
2����
��3�����������ֽܷ����ɴ����������Ҫ��������Һ�д����������������Ҫ��ô���������������������Һ����Ϊ100g���������������Ϊ��100g��19%=19g��ˮ������Ϊ��100g-19g=81g�������ɵĴ��������Ϊx������
2CH
3COOOH=2CH
3COOH+O
2��
152 120
19g x
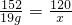
x=15g
����������Һ�д������������Ϊ��

=15.6%
��4���پݷ���ʽ����ԭ���غ㣬����NaCl��
��A������ɱ���Ĺ����Ƿ���������ԭ��Ӧ�Ĺ��̣���A��ȷ��
B��C
3O
3N
3Cl
2Na����Է�������Ϊ12��3+16��3+14��3+35.5��2+23=220����B����
C��C
3O
3N
3Cl
2Na��C��OԪ�ص�������Ϊ
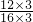
=
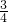
����C��ȷ��
D��ClO
2��NaClO��C
3O
3N
3Cl
2Na�о�����Ԫ�أ�ClO
2����Ԫ�ص���������Ϊ
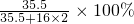
��53%���D��ȷ��
�ʴ�Ϊ����1���ռ��������ƣ�
��2��2H
2O
2
2H
2O+O
2��
��3��2CH
3COOOH=2CH
3COOH+O
2����15.6%��4����NaCl��ACD��
��������1�����������׳��ռ�������ƣ�2����������д��ѧ����ʽ��3�����������ֽܷ����ɴ����������Ҫ����������������������Ҫ��ù����������������4���ٸ��ݷ���ʽ����ԭ���غ���н�𣻢ڸ��ݷ���ʽ�ĺ�����
���������⿼���˳������ʵ���;���й��������������ļ��㼰��ѧʽ�ĺ����֪ʶ�㣬��һ���ۺ��⣮

 2H2O+O2����
2H2O+O2����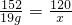
 =15.6%
=15.6%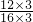 =
=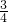 ����C��ȷ��
����C��ȷ��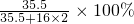 ��53%���D��ȷ��
��53%���D��ȷ�� 2H2O+O2��
2H2O+O2��


 �������ڹ�������������������ʱ��������Ҫ�����ã�
�������ڹ�������������������ʱ��������Ҫ�����ã�