����ϸ�Ķ����ģ�Ȼ��Ҫ��д���йط�Ӧ�Ļ�ѧ����ʽ��
ʳ����Ҫ�ɷ��Ǵ��ᣨ��ѧʽΪCH3COOH �����仯ѧ���ƽ����ᣬ��һ����;�㷺���л��ᣮͨ������£�������������һ����ǿ�Ҵ̼�����ζ����ɫҺ�壬������ˮ�;ƾ�����ˮ��Һ�У�����ֻ�ܽ���������Ӻ���������ӣ�CH3COO-���������ܸ����ý��������û���Ӧ�������������磬��п��Ӧ�Ļ�ѧ����ʽ�ɱ�ʾΪ��Zn+2CH3COOH�T��CH3COO��2Zn+H2����
��1������д������ֱ������������Һ������ͭ��Ӧ�Ļ�ѧ����ʽ��
��2���û�ѧ����ʽ˵��Ϊʲô���õ���������������ʢ�Ż�����ʳ�ף�
��3������һ�ֻ��ý��������Ļ�ѧ���ʽ�Ϊ���⣬�������ܸ����ᷴӦ�������Ȼ��������������ܸ��ȵ���������ˮ��Һ��Ӧ����ƫ�����ƣ�NaAlO2��������������д���÷�Ӧ�Ļ�ѧ����ʽ��
���𰸡�
���������ȸ��ݷ�Ӧԭ���ҳ���Ӧ��������Ӧ���������ݻ�ѧ����ʽ����д���������������д���ɣ�
����⣺��1�����������������Һ��Ӧ���ɴ����ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ��CH
3COOH+NaOH�TCH
3COONa+H
2O������������ͭ��Ӧ���ɴ���ͭ��ˮ����Ӧ�Ļ�ѧ����ʽΪ��2CH
3COOH+CuO�T��CH
3COO��
2Cu+H
2O��
��2���������⣬�����ܸ����������ﷴӦ��������ý��������û���Ӧ������������������������������Ĥ������ᷴӦ���ɴ�������ˮ����������ᷴӦ���ɴ���������������Ӧ�Ļ�ѧ����ʽΪ��6CH
3COOH+Al
2O
3�T2��CH
3COO��
3Al+3H
2O��6CH
3COOH+2Al�T2��CH
3COO��
3Al+3H
2��
��3�����ܸ��ȵ���������ˮ��Һ��Ӧ����ƫ�����ƣ�NaAlO
2������������Ӧ�Ļ�ѧ����ʽΪ��2Al+2NaOH+2H
2O
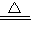
2NaAlO
2+3H
2����
�ʴ�Ϊ����1��CH
3COOH+NaOH�TCH
3COONa+H
2O��2CH
3COOH+CuO�T��CH
3COO��
2Cu+H
2O��
��2��6CH
3COOH+Al
2O
3�T2��CH
3COO��
3Al+3H
2O��6CH
3COOH+2Al�T2��CH
3COO��
3Al+3H
2����
��3��2Al+2NaOH+2H
2O
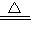
2NaAlO
2+3H
2����
�����������ѶȲ�����ѧ�����ݷ�Ӧԭ����д��ѧ����ʽ����������ѧ����ʽ��д�������ֵĴ����в����Ͽ���ʵ�������������غ㶨�ɡ���д������������ŵȣ�

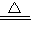 2NaAlO2+3H2����
2NaAlO2+3H2����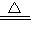 2NaAlO2+3H2����
2NaAlO2+3H2����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�