
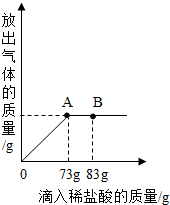
 ��̼���ƺ��Ȼ��ƵĻ���������ˮ�ܽ⣬�Ƴ�131.4����Һ���������μ�������������Ϊ10%��ϡ���ᣬ�ų��������������������ϡ�����������ϵ������ͼ��ʾ�����μ�ϡ������ͼ��B��ʱ������Һ���������������������ճƵù��������Ϊ25.6�ˣ����������ش����⣺
��̼���ƺ��Ȼ��ƵĻ���������ˮ�ܽ⣬�Ƴ�131.4����Һ���������μ�������������Ϊ10%��ϡ���ᣬ�ų��������������������ϡ�����������ϵ������ͼ��ʾ�����μ�ϡ������ͼ��B��ʱ������Һ���������������������ճƵù��������Ϊ25.6�ˣ����������ش����⣺���� ����̼���ƺ����ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼�����ݲμӷ�Ӧ������������������ɶ�����̼�������Ͳμӷ�Ӧ��̼���Ƶ���������������ԭ��������������ȫ��Ӧ��������Һ�����ʵ�����������
��� �⣺�����ɶ�����̼������Ϊx�������Ȼ��Ƶ�����Ϊy���������̼���Ƶ�����Ϊz��
73g��10%=7.3g
Na2CO3+2HCl=2NaCl+H2O+CO2����
106 73 117 44
�⣺�����ɶ�����̼������Ϊx�������Ȼ��Ƶ�����Ϊy���������̼���Ƶ�����Ϊz��
73g��10%=7.3g
Na2CO3+2HCl=2NaCl+H2O+CO2����
106 73 117 44
z 7.3g y x
$\frac{73}{7.3g}$=$\frac{106}{z}$=$\frac{117}{y}$=$\frac{44}{x}$
x=4.4g
y=11.7g
z=10.6g
��2��ԭ����������Ϊ��25.6g-11.7g+10.6g=24.5g��
��3��ǡ����ȫ��Ӧ��������Һ�����ʵ���������Ϊ��$\frac{25.6g}{131.4g+73g-4.4g}$��100%=12.8%��
�ʴ�Ϊ����1��4.4g��
��2��24.5g��
��3��ǡ����ȫ��Ӧ��������Һ�����ʵ���������Ϊ12.8%��
���� ����ϺõĿ���ѧ������ͼ����������ѧ��Ӧ��������ѧ��Ӧ��ȷͼ���еĹؼ��㼰�ߵı仯����ʾ�ĺ��壬��ͼ��ͻ�ѧ��Ӧ���ܽ���ǽ���Ĺؼ����ڣ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 | |
| �ܽ��/g | KNO3 | 13.3 | 31.6 | 63.9 | 110 | 169 | 246 |
| NaCl | 25.7 | 36.0 | 36.6 | 37.3 | 38.4 | 39.8 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | һ��ľ�Ǵ������к���22��ԭ�� | B�� | ľ�Ǵ������л������� | ||
| C�� | ľ�Ǵ���̼���⡢������Ԫ����� | D�� | ľ�Ǵ�����Է�������Ϊ152 g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
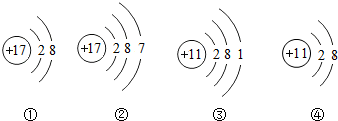
| A�� | �ۢܵĻ�ѧ�������� | |
| B�� | �ڢܵ�ԭ����ͬһ���� | |
| C�� | ����һ�������� | |
| D�� | �٢��γɵĻ������������ĸ�����Ϊ1��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �û���̿����Ӳˮ | |
| B�� | �ó����ʯ��ˮ��ȼ�ŵ�ľ�����������Ͷ�����̼��ƿ��ɫ���� | |
| C�� | �õ�ȼ�ķ�����ȥ������̼�е�����һ����̼ | |
| D�� | ��Ba��OH��2��Һ����Na2SO4��Mg��NO3��2����NH4��2SO4��NaCl������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | K+��Ba2+��OH-��Cl- | B�� | H+��Ba2+��Cl-��NO3- | ||
| C�� | K+��OH-��Cl-��CO32- | D�� | H+��Ag+��Ba2+��NO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com