
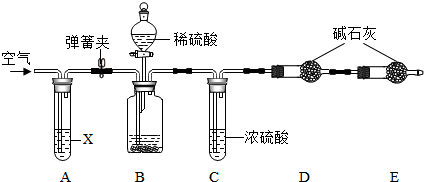
���� ��1������װ�õ��ص㼰ʵ��Ŀ�ģ��������������һ������Ŀ�ģ�����ʵ��Ŀ��������װ��A�м�Һ�����ã�
��2������װ��C�Բⶨ�����Ӱ�죻
��3�����Ը��ݿ����еijɷ�������װ��E������װ�����������ã�
��4������ʵ��ǰ��װ��D�����仯�����ɴ������������Ʒ��̼���Ƶ������������Ʒ��̼���Ƶ�����������
��� �⣺��1��Ϊ�˼�Сʵ��������ȡ�˹�������ķ������Ѳ�����װ��B�ж�����̼ȫ����D�м�ʯ�����գ�ʵ������ȷ��
�������֪������ͨ���ⶨ������̼���������ⶨ̼���Ƶ����������ģ�����Ҫ�ų������еĶ�����̼����ʵ����������A��װ�˼�����Һ�����տ����еĶ�����̼��
��2��Ũ���������ˮ�ԣ���Cװ����������Bװ���ų������л��е�ˮ�����������˴�װ�����ʹ�����е�ˮ������Dװ���м�ʯ�����գ���ʹ�ⶨ����ƫ��
��3�����Dװ��ֱ��������������ͨ��������е�ˮ�����Ͷ�����̼�����Dװ�ö��Բⶨ�������Ӱ�죬����װ��E���������Ƿ�ֹ������ˮ�����Ͷ�����̼����װ��D�У�
��4����Ӧ�зų�������̼���������=85.6g-83.4g=2.2g
��ų�2.2g������̼����̼���Ƶ�����Ϊx
Na2CO3+H2SO4�TNa2SO4+H2O+CO2��
106 44
x 2.2g
$\frac{106}{x}$=$\frac{44}{2.2g}$
x=5.3g
������ƷNa2CO3����������$\frac{5.3g}{6g}$��100%=88.3%��
�ʴ�Ϊ����1��ʹB�в����Ķ�����̼ȫ������D�У�������Һ��
��2��ƫ��
��3����ֹ������CO2��ˮ��������D�У�
��4��88.3%��
���� ���⿼���˺����Ƽ�Ļ������̡���Ӧ�����Ŀ��ơ�ԭ�ϵ�ѭ�����ã��Ƽ�������ֳ�������Ϊ�����Ƽ�������Ƽ����Ӧ�ķ���ʽ�������̵ȷ���Ϊ��������ȵ㣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �ۢ� | C�� | �ڢܢ� | D�� | �ۢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ�| A�� |  | B�� |  | C�� |  | D�� |  |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H2��� | B�� | ̿����� | C�� | CO��� | D�� | һ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ԭ������С�����ӣ������ٷ� | |
| B�� | ԭ���ڻ�ѧ�仯�еı�����Ҫ����ԭ�ӵ����������������� | |
| C�� | ����ͬԪ����ɵ�����һ���Ǵ����� | |
| D�� | ����ԭ��������С����Ҫ�����Ӻ͵��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com