
 ��Щ�������������������ƵĻ�ѧ���ʣ�����������ȴ������ͬ����Ca��OH��2��Ba��OH��2���ܽ�Ⱦʹ���һ���IJ��죬���±���ʾ��
��Щ�������������������ƵĻ�ѧ���ʣ�����������ȴ������ͬ����Ca��OH��2��Ba��OH��2���ܽ�Ⱦʹ���һ���IJ��죬���±���ʾ��| �¶�/�� | 0 | 40 | 80 | |
| �ܽ��/g | Ca��OH��2 | 0.187 | 0.141 | 0.094 |
| Ba��OH��2 | 1.67 | 8.22 | 101.4 | |


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2010?�人����Щ�������������������ƵĻ�ѧ���ʣ�����������ȴ������ͬ����Ca��OH��2��Ba��OH��2���ܽ�Ⱦʹ���һ���IJ��죬���±���ʾ��
��2010?�人����Щ�������������������ƵĻ�ѧ���ʣ�����������ȴ������ͬ����Ca��OH��2��Ba��OH��2���ܽ�Ⱦʹ���һ���IJ��죬���±���ʾ��| �¶�/�� | 0 | 40 | 80 | |
| �ܽ��/g | Ca��OH��2 | 0.187 | 0.141 | 0.094 |
| Ba��OH��2 | 1.67 | 8.22 | 101.4 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
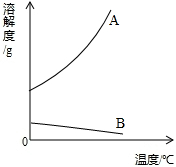
��Щ�������������������ƵĻ�ѧ���ʣ�����������ȴ������ͬ����Ca(OH)2��Ba(OH)2���ܽ�Ⱦʹ���һ���IJ��죬���±���ʾ��
| �¶�/�� | 0 | 40 | 80 | |
| �ܽ��/g | Ca(OH)2 | 0.187 | 0.141 | 0.094 |
| Ba(OH)2 | 1.67 | 8.22 | 101.4 |

����������Ϣ�ش��������⣺
(1)ͼ�У���ʾCa(OH)2�ܽ�����ߵ���________(�A����B��)��
(2)���Ҫ��Ca(OH)2�IJ�������Һת��Ϊ������ Һ���ɲ�ȡ�ķ�����____________(ֻ��һ��)��
Һ���ɲ�ȡ�ķ�����____________(ֻ��һ��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���人 ���ͣ������
| �¶�/�� | 0 | 40 | 80 | |
| �ܽ��/g | Ca��OH��2 | 0.187 | 0.141 | 0.094 |
| Ba��OH��2 | 1.67 | 8.22 | 101.4 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�п����� ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��3�֣���Щ�������������������ƵĻ�ѧ���ʣ�����������ȴ������ͬ����Ca(OH)2,Ba(OH)2���ܽ�Ⱦʹ���һ���IJ��죬���±���ʾ��
| �¶�/�� | 0 | 40 | 80 | |
| �ܽ��/g | Ca(OH)2 | 0.187 | 0.141 | 0.094 |
| Ba(OH)2 | 1.67 | 8.22 | 101.4 | |
����������Ϣ�ش��������⡣
��1�� ��ͼ�У���ʾCa(OH)2�ܽ�����ߵ���-----------( �A����B��)��

��2�� ���Ҫ��Ca(OH)2�IJ�������ҺתΪ������Һ���ɲ�ȡ�ķ�����--------------��ֻ��һ�֣�
��3�� ���г���ʱ��Ca(OH)2,Ba(OH)2�������ʵ�ij������Һ����Ҫ�õ��ϴ�����Ba(OH)2��Һ����ȡ����������Ϊ-----------------��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com