
��ѧ������һ����ͨ�õķ��ţ������DZ��ﻯѧ����Ĺ��ߡ�
��1���û�ѧ������д�±��ո�
��2����ԭ��_________����2��������_________����2��������_______��
��2��д�����л�ѧ���������֡�2�������壺
��2NO �� ��2Na+ ��
��Ca2+ �� ��H2O ��
��3�����ӽṹʾ��ͼ�ɼ���������ر�ʾ���ӵĽṹ����������ͼʾ�ش����⣺Aͼʾ��ʾԭ�ӣ���������ϵ�����Ϊ_________�������֣����������������б�ʾͬ��Ԫ�ص���_________������ţ��� 
A B C D
��1����2O ��2O2 ��2O2- ��2����2��һ���������� ��2�������� ��һ�������Ӵ�������λ����� ��һ��ˮ��������������ԭ�ӣ�3��6 B C
���������������1����ԭ�ӷ�����Ԫ�ط�����ͬ��ԭ�ӵĸ���д��ԭ�ӷ��ŵ�ǰ�棬��2����ԭ�ӣ�2O����һ������������������ԭ�ӹ��ɣ���2�������ӣ�2O2��һ�������Ӵ�������λ�ĸ���ɣ���2�������ӣ�2O2-��2���ٻ�ѧʽǰ������ֱ�ʾ���Ӹ�������2NO��ʾ2��һ���������ӣ������ӷ���ǰ������ֱ�ʾ���ӵĸ�������2Na+��ʾ2�������ӣ������ӷ��ŵ����Ͻǵ����ֱ�ʾһ�����Ӵ��ĵ��������Ca2+��ʾһ�������Ӵ�������λ����ɣ��ܻ�ѧʽ�е����ֱ�ʾһ�����Ӻ���ԭ�Ӹ�������H2O��ʾһ��ˮ��������������ԭ�ӣ���3��ԭ�������������ں������������Aͼʾ��ʾԭ�ӣ���������ϵ�����Ϊ6����������ͬ��ʾͬ��Ԫ�أ������������б�ʾͬ��Ԫ�ص���B C��
���㣺Ԫ�ط��ż���Χ���ֵ����塢ԭ�ӵĽṹ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ����̼��������̼�������������Ͷ���������ѡ���ʵ��������û�ѧʽ��գ�
��1��������̬�����Ҿ��п�ȼ�Ե������� ��
��2���ɳ�����Ƶ������� ��
��3������������������� ��
��4����������Ѫ�쵰��ϵ��ж������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ѧ֪ʶϵͳ���������ڶ��������ʶ��������������йط�����������ۡ�
��1����������ɸ�������ɺ����ʽ��з��࣮��K��H��O��N����Ԫ��������ѡ��������ɺ��ʵ����ʣ����仯ѧʽ�ֱ�����ͼ1�Тڡ��ĺ����ϡ�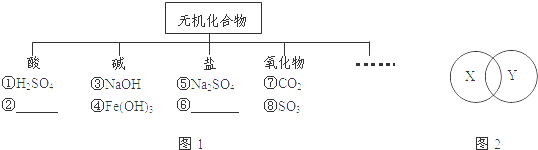
�뽫����ۡ������ּ�������࣬�ɷ�Ϊ�� ����
��2����ѧ��Ӧ֮�䡢��ѧ����֮����а��������С�����ȹ�ϵ���±���X��Y����ͼ2��ʾ��ϵ��
���� ����ѡ�����б���ѡ���
| | A | B | C | D |
| X | ���Ϸ�Ӧ | �û���Ӧ | ������ | ���� |
| Y | ������Ӧ | ���ֽⷴӦ | ������ | ̼���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ����������β����Ӧ���۹��̡�ͼ�е����� �������ƣ�����ѧ����ʽΪ �����ɵĵ����뻯�����������Ϊ ��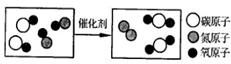
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ����������ǽ����������������������ϵ��
��1���û�ѧ���ű�ʾ����������ԭ�ӡ����� ����
��2�����ж��ڻ�ѧʽ��H20���ĸ��ֱ�����ȷ������ ��
A����ʾˮ�������� B����ʾˮ��������Ԫ�غ���Ԫ�����
C����ʾ1��ˮ���� D����ʾˮ������2����ԭ�Ӻ�1����ԭ�ӹ���
��3�������ѵĻ�ѧʽΪC2H6O���������� ����Ԫ����ɣ������⡢��Ԫ�ص�������Ϊ�� ������д��������ȣ�
��4��A��B��C��D�ֱ��ʾ4�����ʣ��������ʵķ��ӵ���ʾ��ͼ��ͼ��ʾ��
| ���� | B | C | D | ͼ�� |
| �� ʾ��ͼ |  |  |  |  |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
д�����з�Ӧ�Ļ�ѧ����ʽ������������ע����Ӧ���ͣ�
��1�����ڿ����б������γ�һ�����ܵı�Ĥ��
���� ����Ӧ��
��2��ʵ���ҳ��ü�������غͶ������̵Ļ������������
���� ����Ӧ��
��3��ʵ���ҳ���ϡ���������ʯ��Ӧ����ȡ������̼��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1�����ʵ��ķ��ź������Լ���ѧʽ���
�ٱ���ˮ�Ļ�ѧ���ʵ���С���� ������ͭ�е�������
�۹�����������Ԫ�صĻ��ϼ� ������� �������
��2��д�����з�Ӧ�Ļ�ѧ����ʽ
��ʵ�����ù���������ȡ����
��ľ̿���»�ԭ����ͭ
���д̼�����ζ�������ɵĻ��Ϸ�Ӧ
����ˮ���ĵĻ��Ϸ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�û�ѧ���ű�ʾ��
��1�������к�����ߵ�Ԫ�� ��
��2������ͭ��ͭԪ�صĻ��ϼ�Ϊ+2�� ��
��3������KClO3��������Ĺ��ɺͻ��ϼۣ�������ƵĻ�ѧʽΪ ___________��
��4��ˮͨ��ֽ�ķ��ű���ʽ ��
��5��þ���ڿ�����ȼ�յķ��ű���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ִ�����X��Y�ڿ�����ȼ��ʱ�Ļ�ѧ����ʽ���£���Ӧ����ʡ�ԣ���
x+3O2=2CO2+3H2O��2y+5O2=4CO2+2H2O
��1��д��x��y�Ļ�ѧʽ��x -��y - ��
��2��x��y�ж����е�Ԫ���� ��֤������Ԫ�ص�ʵ�鷽���ǣ�
��
��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com