������CuSO
4?5H
2O������Ҫ�Ļ���ԭ�ϣ���ҵ����Cu��ŨH
2SO
4��ŨHNO
3��Ӧ�Ʊ������ʣ���ȡ��Ъ���ȡ�����ŨHNO
3�ķ������Ʊ�CuSO
4?5H
2O���¹��գ�ģ���Ʊ�װ����ͼ��ʾ����������ش��������⣮
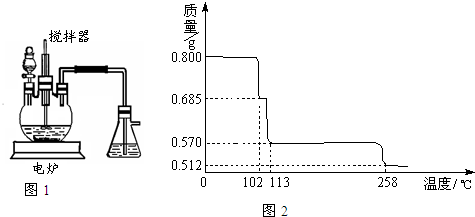
��1����ͼ1װ���У���Һ©����װ��Һ����
Ũ����
Ũ����
���Ũ���ᡱ��Ũ���ᡱ�����÷�Ӧ������ж�����NO��NO
2�ȣ���ƿ��Һ��������ո����壬��Ӧԭ��Ϊ��NO+NO
2+2NaOH=2NaNO
2�������ʻ�ѧ����
��������
��������
��+H
2O����װ���н�������������
��ֽӴ����ӿ췴Ӧ����
��ֽӴ����ӿ췴Ӧ����
����Ӧ���ò�Ʒ��
������ƿ
������ƿ
���������ƿ������ƿ�����У�
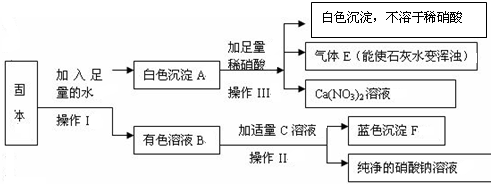
��2�����ͭ�ۡ����ἰ���ᶼ�Ƚϴ��������Ƶõ�CuSO
4?5H
2O�г������е�������һ�������Σ��仯ѧʽ��
Cu��NO3��2
Cu��NO3��2
����֪10�桢20��ʱ�й����ʵ��ܽ��Ϊ��CuSO
4?5H
2O��17.4g��20.7g�����������Σ�95.3g��125.1g�����ᴿCuSO
4?5H
2O��ʵ�������
�ؽᾧ������ȴ�ᾧ��
�ؽᾧ������ȴ�ᾧ��
��
��3������ˮ����ͭ����ij˫��ˮ���Ƿ�ˮʱ�����˷��ֹ�������⣬�����ָ�˫��ˮ�������ݲ������Դ�������IJ�����
ͭ������������˫��ˮ�ֽ������
ͭ������������˫��ˮ�ֽ������
������������ʵ�������
�ô�����ľ�����飬���Ƿ�ȼ
�ô�����ľ�����飬���Ƿ�ȼ
��
��4����0.80g CuSO
4?5H
2O��Ʒ������ˮ���̵��������ߣ���Ʒ�������¶ȱ仯�����ߣ���ͼ2��ʾ��
����ͼ2�п��Կ�����CuSO
4?5H
2O�ֽ������¶���
102��
102��
��
����ͨ������ȷ��238��ʱ�������ʵĻ�ѧʽ����Ҫ������̣�
�۽�CuSO
4?5H
2O������ȵ�570��ֽ�õ�����Ҫ�����Ǻ�ɫ��ĩ���������������ˮ�������÷�Ӧ�Ļ�ѧ����ʽΪ
CuSO
4?5H
2O
CuO+SO
3��+5H
2O��
CuSO
4?5H
2O
CuO+SO
3��+5H
2O��
��



 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д� ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д�