
�����������ڰ�����ʦ������ѧʵ����ʱ������һƿ��Ϊ����İ�ɫ���塣��ϸ�鿴ҩƷ��ǩ��ȷ���仯ѧʽΪH2C2O4��Ϊ�ˣ���������չ����̽�����
���������ϡ�
�Ų������Ҷ�����׳ƣ�������л���Ԫ�ᣬ�ṹ��ʽΪHOOCCOOH�����ᾧ��������ˮ�������Ƿ�ˮ������Ȼ���еĺܶ�ֲ���粤�˵ȶ��������ᡣ
�Ʋ���ȶ�����189.5��ʱ��ֽ⡣
�Dz���������ͨ�ԣ������Աȴ���ǿ10000�������γɵ����У�ֻ�����Ρ�����������ˮ��
�ȶ�������ʯ�ࣨ��CaSO4����±ˮ����CaCl2�����㡱��ֲ�ﵰ�����̶��ɣ��ʶ����к��д����ĵ����ʺơ�
��������⡿
����һ������ֽ�IJ�������Щ��
���������䴫˵�������ܺͲ���һ��ʳ�ã��������ɡ�����ʧ������Ŀ����أ�
���������衿
�������һ������Ϊ�����������غ㶨�ɣ�����ֽ�IJ������Ӧ��ΪCO2��H2O��
����ȴ��Ϊ�����ȵļ���������Ե����⡣����������ѧϰ����֪ʶ�� �� �ķֽ�����ΪCO2��H2O������������Ϊ����ֽ�IJ�����Ͽ���Ϊ �� ��
��ʵ������ۡ�
Ϊ��˵�����ȣ�������������µ�ʵ�飬����������һ������ʵ���龰�ɡ�
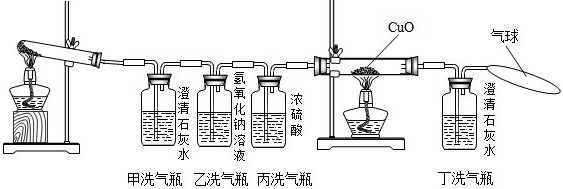
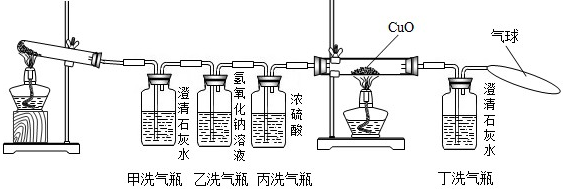
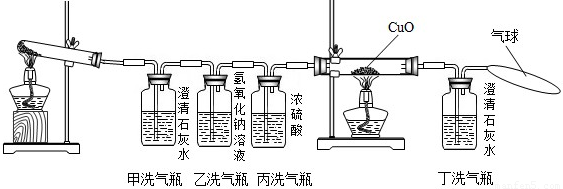
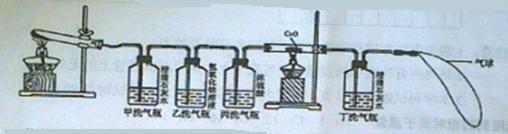 �����������ᾧ��װ��Ӳ���Թܺ�ȼ�˾ƾ��ƣ�һ����Թ�ǰ���������˺ܶ���ɫ��СҺ�Ρ�������֤������ֽ������ �� ��
�����������ᾧ��װ��Ӳ���Թܺ�ȼ�˾ƾ��ƣ�һ����Թ�ǰ���������˺ܶ���ɫ��СҺ�Ρ�������֤������ֽ������ �� ��
�������ڼ�ƿ��װ���˳���ʯ��ˮ������ƿ��װ����Ũ�Ƚϴ��NaOH��Һ���ڱ�ƿ��װ����Ũ���ʵ��ʱ��ƿ�г��ְ�ɫ���ǣ�˵������ֽ������ �� ��ʹ����ƿ��Ŀ���� �� ��
��������˫ͨ����װ���˺�ɫ��CuO��ĩ������ȼ�˾ƾ��ƣ���������ij������룬��ɫ��ĩ��Ϊ��ɫ���Ҷ�ƿ�г���ʯ��ˮ����ǡ����ͺ�ɫ��ĩ���ɫ�Ļ�ѧ����ʽ�� �� ��˫ͨ�ܺͶ�ƿ��������ͬ˵������ֽ������ �� ����ƿ�������һ����������Ϊ�� �� ��
����������������ֽ�Ļ�ѧ����ʽ�� �� ��
�����������еĴ�˵�������Ľ���Ӧ���� �� ��
 ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�п����� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ�������п���ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com